- Department of Neurosurgery, University Hospitals Leuven, Herestraat, Leuven, Belgium.
DOI:10.25259/SNI_377_2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Jan De Vlieger, Joost Dejaegher, Frank Van Calenbergh. Multidimensional, patient-reported outcome after posterior fossa decompression in 79 patients with Chiari malformation type I. 13-Dec-2019;10:242
How to cite this URL: Jan De Vlieger, Joost Dejaegher, Frank Van Calenbergh. Multidimensional, patient-reported outcome after posterior fossa decompression in 79 patients with Chiari malformation type I. 13-Dec-2019;10:242. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=9805
Abstract
Background: We studied patient-reported outcome among patients who underwent posterior fossa decompression (PFD) for Chiari malformation type I (CM-I).
Methods: We interviewed patients who underwent PFD for CM-I from 1995 to 2016.
Results: A total of 79 patients were interviewed. The median age at surgery was 30 years (range 5–72 years) with 27 pediatric patients. Forty-six patients had syringomyelia (36 adults and 10 pediatric patients). Fifty-four patients (68%) reported at least some improvement, 46 (58%) important improvement, 13 (16%) worsening, and 12 stabilization (15%). Any improvement as well as important improvement were significantly more often reported in the nonsyringomyelia group (85% vs. 57%, P = 0.01 and 76% vs. 46%, P = 0.01, respectively). Of the 47 patients reporting preoperative neck pain, 31 (66%) reported at least some improvement after surgery and 9 (19%) worsening after surgery. Of the 59 patients experiencing headaches before surgery, 45 (76%) reported at least some improvement after surgery and 4 (7%) worsening. Quality of life was mostly affected by pain and discomfort in all groups. Sixty-two patients (78%) were satisfied or very satisfied with the results of surgery and 8 (11%) were unsatisfied or very unsatisfied. Up to 71 patients (90%) would consent to surgery again.
Conclusion: In CM-I patients, PFD offers symptom improvement in about two-thirds of patients with high patient satisfaction. Symptom improvement is significantly higher in patients without associated syringomyelia, but patient satisfaction is similar. Symptom worsening is more frequent in the adult than in the pediatric population, with similar rates of postoperative improvement and patient satisfaction.
Keywords: Chiari malformation type I, Outcome, Patient-reported outcome, Patient satisfaction
INTRODUCTION
Symptomatic Chiari malformation type I (CM-I) is a relatively rare disorder characterized by a hindbrain malformation resulting in tonsillar invagination in the spinal canal and crowding of the neural structures at the level of the foramen magnum. Local compression can induce cerebellar, spinal, or brainstem symptoms. Cerebrospinal fluid obstruction can cause hydrocephalus and craniospinal pressure dissociation, inducing Valsalva-induced headaches and/or syringomyelia. Posterior fossa decompression (PFD) for symptomatic CM-I with and without syringomyelia is a well-established treatment to restore cerebrospinal fluid flow across the foramen magnum[
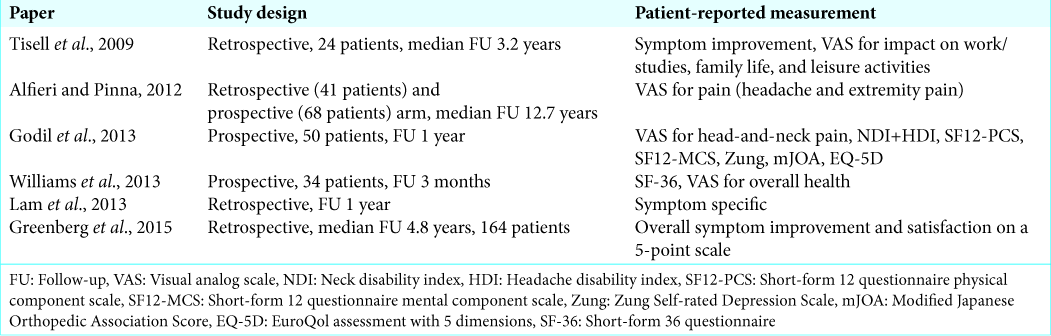
Patient-reported outcome measures (PROMs) can be evaluated in different ways. In a symptom-specific outcome evaluation, the evolution of the presenting symptoms is measured. In an overall symptom outcome assessment, patients are asked to describe an overall impression of change. In a quality-of-life assessment, patients are asked to report on several aspects of daily life and the extent in which the studied condition limits them in those aspects.
PROMs can be generic, which means applicable to multiple different conditions, or disease-specific, meaning that they are developed for specific diseases or conditions. For CM-I, there is only one disease-specific PROM, the Chicago Chiari Outcome Scale, which is a retrospective physician-reported outcome scale. Validated PROMs for CM-I are the Neck Disability Index, Euro-Qol-5d, Zung Self-rating Depression Scale, and the short form-12,[
Surgery for CM-I is aimed at reducing syrinx size and/or alleviating symptoms attributed to the craniocervical crowding of neural tissue. Given the often subjective symptoms in Chiari I, interventions aimed at treating this condition are particularly interesting to evaluate with PROMs. Ideally, these would consider multiple facets of patients’ well-being and would be evaluated over a long period of time because the symptoms are often related to discomfort and pain, and the condition is often diagnosed in the pediatric population or in the fifth decade[
The same meta-analysis demonstrated that in almost all studies, neurological outcome is reported by the authors.[
Although prospective, multidimensional, and long-term patient reporting would be ideal to evaluate any treatment for CM-I, we did not find any such studies. We suspect that such a study would probably face a fairly high number of patients being lost to follow-up. The aim of our study was to obtain multidimensional PROMs in patients who underwent PFD for symptomatic CM-I and to identify predictors of favorable outcome, as reported by patients. The ultimate goal is better preoperative counseling for patients suffering from symptomatic CM-I in whom this treatment is considered.
METHODS
We retrospectively studied all PFD surgeries for CM-I, with and without syringomyelia, performed at our center from January 1995 to December 2016 to investigate the safety of this procedure. We searched the University Hospital of Leuven’s database for “PFD,” “Chiari,” and “Syringomyelia.” Patients who underwent a first-ever PFD for CM-I were selected for further evaluation. The main author (JDV) reviewed the files for patient gender, age at operation, the duration of symptoms before surgery, the time interval between operation and questionnaire, presence or absence of syringomyelia, any subsequent surgeries for CM-I- related complications or syrinx progression, and extent of decompression and syrinx evolution on postoperative magnetic resonance imaging. Adults were defined as patients older than 18 years at the time of surgery.
We construed a list with generic PROMs. This questionnaire included the Patient Global Impression of Change (PGIC)[
All interviews were performed through telephone by the main author (JDV). Before starting the telephone interview, patients were asked whether they felt that they could still reliably remember their symptoms and the subsequent evolution after surgery and if not, they were excluded from the study. Questionnaires were answered by the patient, also in the pediatric population. The protocol was approved by the ethical board of our hospital (protocol reference mp18865). For statistical analysis, we used the Mann–Whitney U-test for continuous variables and the Fisher’s exact test for nominal variables (GraphPad Prism 8®).
RESULTS
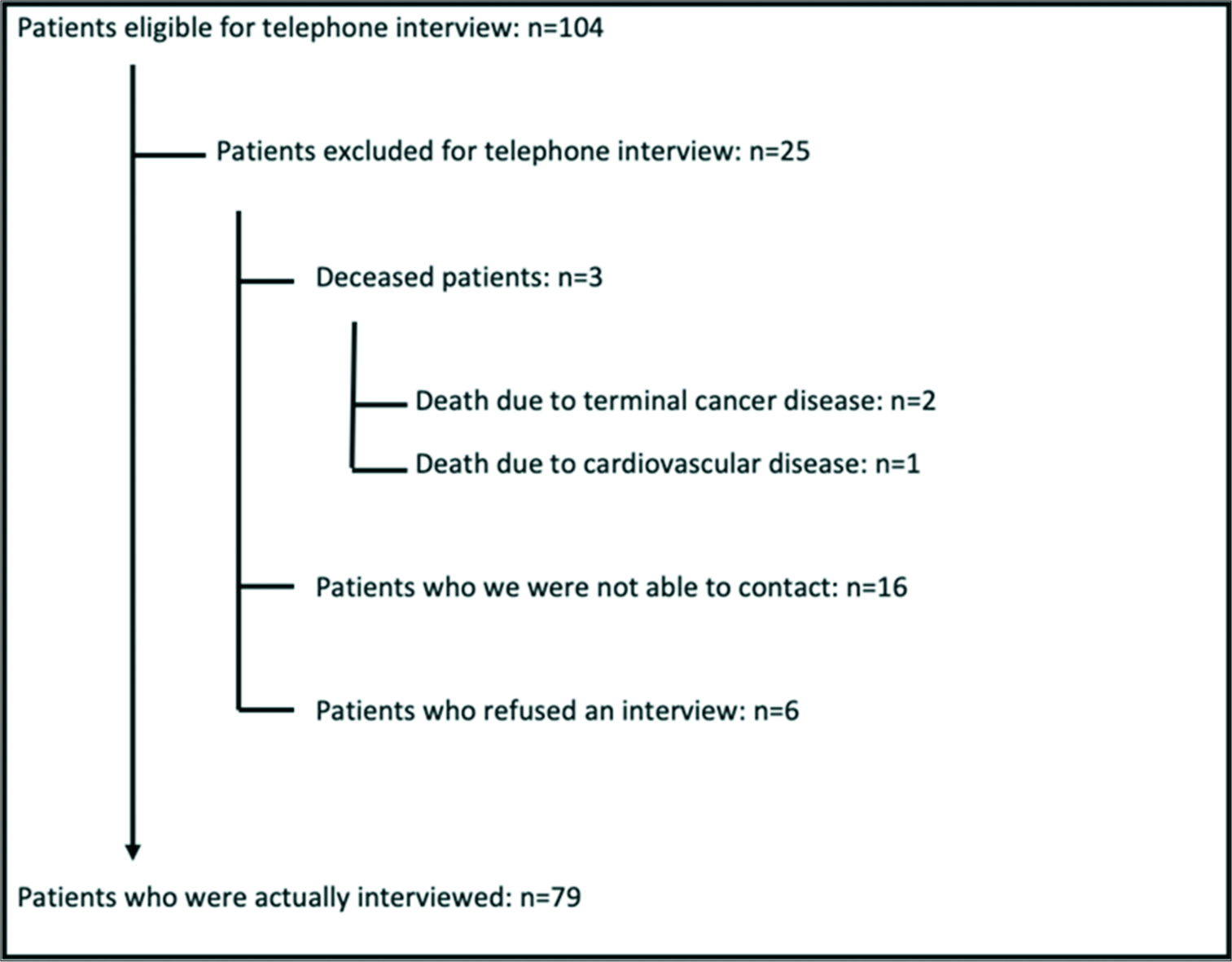
We retrieved 104 patients who were eligible for a telephone interview [
The remaining 79 patients were between 5 and 72 years old with a median age of 30 years, of whom 46 had associated syringomyelia and 52 were adults. Patient characteristics are listed in
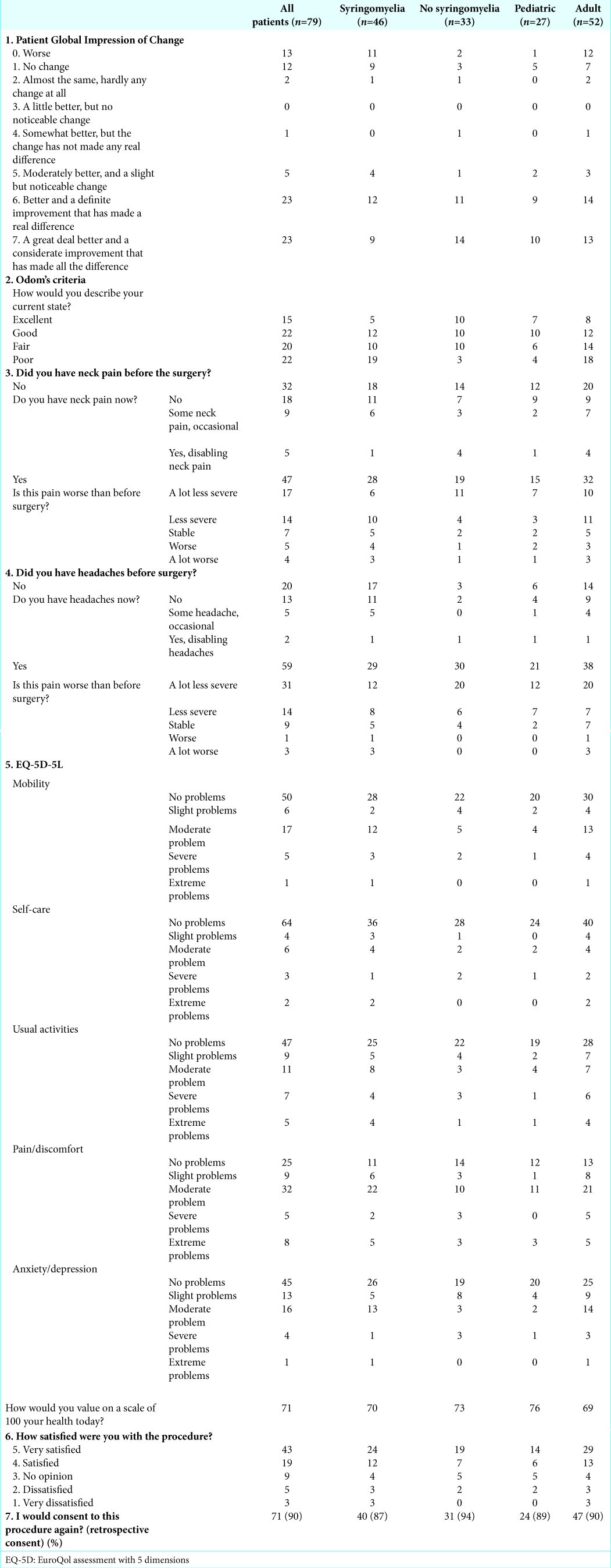
Subjective overall outcome of surgery (PGIC score and Odom’s criteria scores)
Fifty-four patients (68%) reported at least a small improvement of their symptoms defined as PGIC ≥2 and 46 (58%) reported an important improvement defined as PGIC ≥6. Worsening of symptoms was reported by 13 patients (16%) and stabilization by 12 patients (15%).
Postoperative worsening of symptoms was significantly more reported in the adult population than in the pediatric population (23% vs. 4%, P = 0.03) but was not significantly different when comparing the syringomyelia and nonsyringomyelia groups (24% vs. 6%, P = 0.06). The rate of postoperative improvement (any improvement [PGIC ≥2] as well as important improvement [PGIC ≥6]) was not significantly different between the pediatric and adult groups but was significantly different between the syringomyelia and nonsyringomyelia groups (57% vs. 85%, P = 0.01 and 46% vs. 76%, P = 0.01, respectively).
Figure 3:
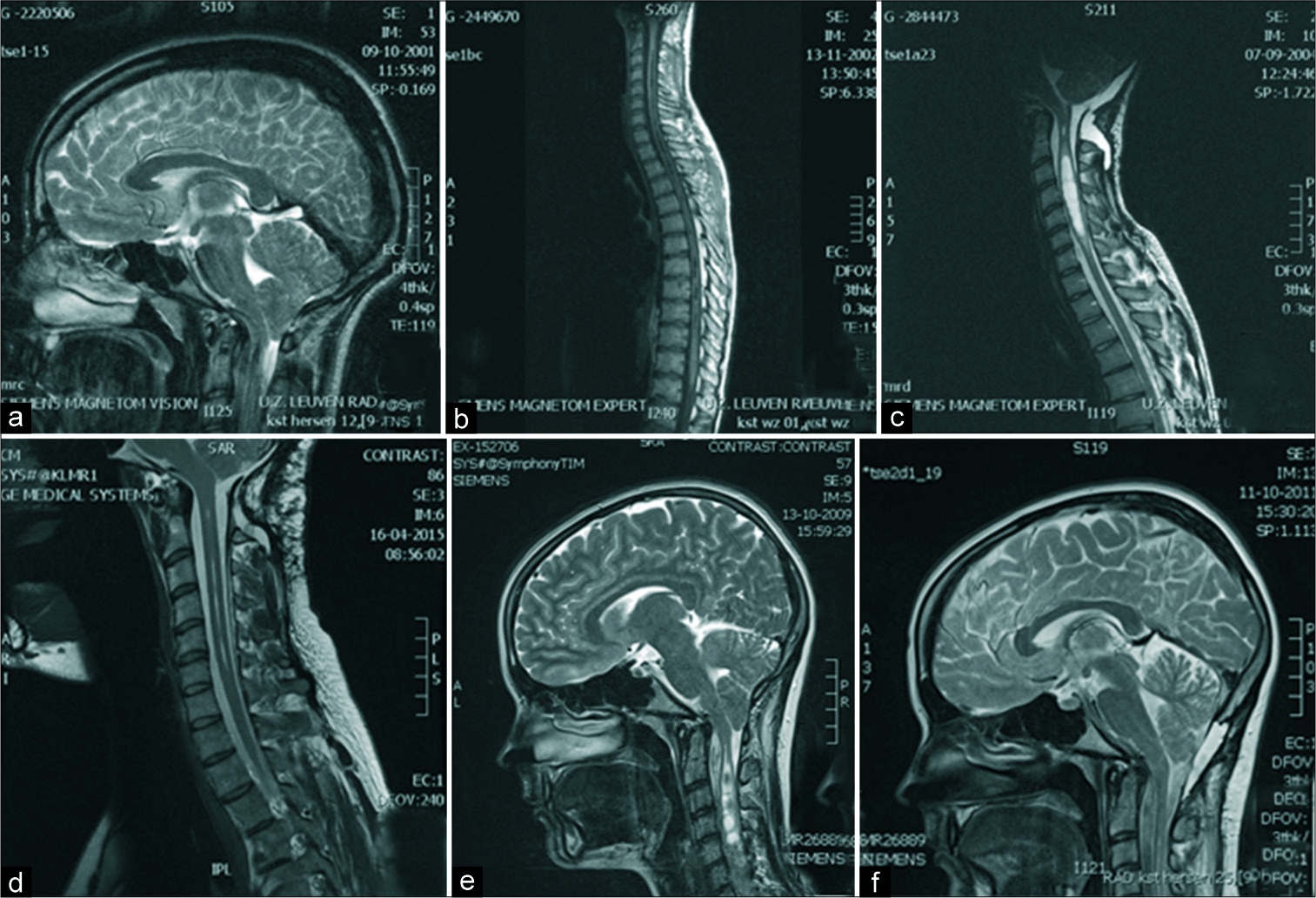
(a-d) Preoperative and postoperative magnetic resonance imaging (MRI) for male patient who was operated, 16 years before the telephone interview, at the age of 20 due to suspended thermoanalgesia for 1 month due to Chiari malformation type I (CM-I) and a syrinx from C1 to T10. He underwent a posterior fossa decompression (PFD) with C1 laminectomy and duraplasty. During the telephone interview, he stated a clear improvement in symptoms (Patient Global Impression of Change 6) and told us he was very satisfied with the surgery and would consent to surgery again. (a) Preoperative MRI demonstrates crowding at the foramen magnum. (b) Postoperative spinal MRI shows initial decrease of the syrinx. (c) Delayed postoperative MRI due to the recurrence of symptoms demonstrates an increase in syrinx size despite adequate decompression. A revision surgery performed 32 months after the first surgery demonstrates extensive adhesions; adhesiolysis and redo duraplasty were performed. (d) Postoperative MRI 10 years after the second surgery shows adequate decompression and a decreased syrinx. (e and f) Preoperative and postoperative MRI for female patient who was operated, 7 years before the telephone interview, at the age of 17 years due to girdle pain for 6 months due to CM-I with a syrinx from C1 to L1. She underwent a PFD with C1 laminectomy and duraplasty. During the telephone interview, she reported stable symptoms since surgery, but no improvement whatsoever. She told us she was satisfied with the surgery and would consent to surgery again. (e) Preoperative MRI demonstrating crowding at the foramen magnum and syrinx in the cervical spinal cord. (f) Postoperative MRI, performed 23 months after surgery, demonstrating adequate decompression and near obliteration of the syrinx. An MRI performed 2 months after surgery had shown similar results.
Of the two nonsyringomyelia patients who reported worsening of the symptoms after surgery, both had a satisfying radiological decompression of the craniocervical junction. One of these patients had an occipitocervical fusion during the PFD procedure due to craniocervical instability. Of the three nonsyringomyelia patients who reported stable postoperative symptoms, all had a good radiological decompression on postoperative imaging. For one of these patients, a revision was performed which demonstrated extensive arachnoid scarring at the surgery site.
Upon comparison of the groups who underwent surgery less (n = 14) or more than 2 years ago (n = 65), we found no significant difference regarding reported important improvement (PGIC >6; 86% vs. 77% respectively, P = 0.72). The same held true for the groups operated upon less or more than 5 years ago (n = 42 and n = 37 respectively, 79% and 78% respectively, P = 1.00).
Head-and-neck pain after surgery
Forty-seven patients stated having had neck pain before surgery. Thirty-one of these (66%) reported at least some postoperative improvement and 9 (19%) described worsening. Syringomyelia and age were not significant for postoperative improvement of neck pain (P = 0.21 and P = 1.00).
Of the 32 patients with no preoperative neck pain, 5 (16%) experienced disabling neck pain after surgery with no significant differences for the presence of syringomyelia or for pediatric patients (P = 0.14 and P = 0.63).
Of the 59 patients with preoperative headaches, 45 (76%) experienced at least some improvement after surgery. Syringomyelia and age group were not significantly predictive for postoperative improvement of headache (P = 0.13 and P = 0.11). In 4 patients (7%), headaches got worse after surgery.
Of the 20 patients who did not have a headache before surgery, 2 (10%) developed disabling headache after surgery, 1 in the adult, and 1 in the pediatric group.
Quality-of-life assessment, EQ-5D-5L
Patient satisfaction
Patient satisfaction was high, with 62 patients (78%) being satisfied or very satisfied with surgery. Only 8 patients (11%) were unsatisfied or very unsatisfied. Up to 71 patients (90%) would consent to surgery again. There was no significant difference with regards to the age group or presence of syringomyelia for high patient satisfaction, overall patient satisfaction, high patient dissatisfaction, overall dissatisfaction, or retrospective consent.
Of the five patients who did not have neck pain before surgery but had disabling neck pain after surgery, three were satisfied with surgery and two had no specific opinion on satisfaction with surgery. Of the nine patients who had neck pain before surgery, five reported worse neck pain (of whom two were very satisfied, two had no specific opinion on satisfaction, and one was very unsatisfied with the surgery) and four reported neck pain that was a lot worse (with two unsatisfied patients, one very unsatisfied patient, and one having no specific opinion).
Both patients who did not have headaches before surgery but had disabling headache after surgery were satisfied with the surgery.
Of the four patients whose headache got worse or a lot worse after surgery, two were satisfied, one was dissatisfied, and one was very dissatisfied with the surgery.
DISCUSSION
We present retrospective, multidimensional, and patient- reported outcomes for PFD in a series of 79 patients suffering from CM-I, of whom 42 had associated syringomyelia. The main focus was overall impression of improvement, evolution of neck-and-head pain after surgery, postoperative quality of life, and patient satisfaction. This is to our knowledge the largest series so far on this subject.
This study demonstrates that in a carefully selected patient population PFD is an effective treatment for symptomatic CM-I patients with the majority of patients reporting at least some improvement (68%) or important improvement (58%). Our results are comparable to the 75% improvement, 16.6% stabilization, and 8.5% worsening rate reported in a recent meta-analysis for the treatment for CM-I, which was mainly based on physician-reported outcome studies.[
In our cohort, rates of subjective important improvement were similar in groups operated longer or less than both 2 years and 5 years ago. This could imply that PFD offers long- term symptom improvement, although a prospective study would obviously be better suited to support this hypothesis.
Surprisingly, patients who did not suffer from neck or head pain before surgery but had disabling neck of head pain after surgery were often satisfied with the overall outcome of the surgery. Patients who experienced worsening of preexisting neck or head pain were often not satisfied. Our hypothesis is that in the first group, the neck-and-head pain is probably reported as bearable in case of improvement of the preoperative symptoms, whereas in the second group, the preexisting pain was probably at least one of the reasons to consider surgery and worsening was considered by the patients to be unsatisfactory.
The quality of life in patients who underwent PFD for CM-I measured by EQ-5D-5L was relatively good. It was mostly affected by pain and discomfort. Adult patients reported significantly more problems with moderate to extreme anxiety and depression as compared to the pediatric patients. We hypothesize that this reflects a general tendency in the population and is not specific to the CM-I population.
An overwhelming majority of patients who have undergone PFD for CM-I would consent to surgery again. This is somewhat remarkable since only two-thirds remarked a clear improvement of symptoms. This might be due to the often progressive nature of CM-I and stabilization of symptoms could possibly be regarded as a good result by the patient, although this is not reflected as such in PGIC or Odom’s criteria scores.
There are some evident limitations to this study. First of all, patient-reported measures are ideally collected prospectively. This would give a clear preoperative patient evaluation of complaints and allow evaluating any evolution in patient- reported outcomes overtime. However, such a study performed over a longer time period would likely be affected by a high dropout rate. For CM-I, a long-term follow-up is important because, in general, patients have to live with the remaining symptoms for decades. The second limitation is the long study interval with a median operation interview interval of 6.3 years. This has possibly induced a recollection bias for patients having undergone surgery a long time ago. Before starting the telephone interview, patients were asked whether they felt that they could still reliably remember their symptoms and the subsequent evolution after surgery and if not, they were excluded from the study. This was the case for only one patient. Furthermore, PROMs are inherently subjective and the goal of this study is to evaluate how the patients feel about the sustained postoperative effects. From this point of view, the sometimes long time interval between surgery and questionnaire is not necessarily a limitation. Last but not least, there is an inevitable inclusion bias in a retrospective study. We only present data from patients we operated on. For instance, a patient suffering from a specific headaches or girdle pain with only a mild Chiari malformation, especially if there is no crowding of the neural structures at the level of the foramen magnum, is not selected for surgery. Good patient-reported outcome scores as in this study may thus also reflect careful patient selection. If the extent of tonsillar herniation through the foramen magnum is the only criteria for surgery, disregarding the symptoms, and/or extent of crowding of the neural structures at the foramen magnum, the patient-reported outcome will most likely not be as good.
CONCLUSION
We present a retrospective PROM in 79 CM-I patients, evaluating overall subjective improvement, evolution of postoperative neck pain and headache, quality of life, and overall patient satisfaction after PFD surgery. In a carefully selected patient population, PFD offers important symptom improvement in about two-thirds of patients with high patient satisfaction rates and a good postoperative quality of life. Symptom improvement is significantly less reported by patients suffering from preexisting syringomyelia, but overall patient satisfaction is similar. We believe that this study can contribute to better preoperative patient counseling.
References
1. Alfieri A, Pinna G. Long-term results after posterior fossa decompression in syringomyelia with adult Chiari Type I malformation. J Neurosurg Spine. 2012. 17: 381-7
2. Arnautovic A, Splavski B, Boop FA, Arnautovic KI. Pediatric and adult Chiari malformation Type I surgical series 1965-2013: A review of demographics, operative treatment, and outcomes. J Neurosurg Pediatr. 2015. 15: 161-77
3. EuroQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990. 16: 199-208
4. Gilmer HS, Xi M, Young SH. Surgical Decompression for Chiari Malformation Type I: An Age-Based Outcomes Study Based on the Chicago Chiari Outcome Scale. World Neurosurg. 2017. 107: 285-90
5. Godil SS, Parker SL, Zuckerman SL, Mendenhall SK, McGirt MJ. Accurately measuring outcomes after surgery for adult Chiari I malformation: Determining the most valid and responsive instruments. Neurosurgery. 2013. 72: 820-7
6. Greenberg JK, Yarbrough CK, Radmanesh A, Godzik J, Yu M, Jeffe DB. The Chiari severity index: A preoperative grading system for Chiari malformation Type 1. Neurosurgery. 2015. 76: 279-85
7. Hurst H, Bolton J. Assessing the clinical significance of change scores recorded on subjective outcome measures. J Manipulative Physiol Ther. 2004. 27: 26-35
8. Klekamp J. Surgical treatment of Chiari I malformation--analysis of intraoperative findings, complications, and outcome for 371 foramen magnum decompressions. Neurosurgery. 2012. 71: 365-80
9. Ladner TR, Westrick AC, Wellons JC, Shannon CN. Health-related quality of life in pediatric Chiari Type I malformation: The Chiari Health Index for Pediatrics. J Neurosurg Pediatr. 2016. 17: 76-85
10. Lam FC, Penumaka A, Chen CC, Fischer EG, Kasper EM. Fibrin sealant augmentation with autologous pericranium for duraplasty after suboccipital decompression in Chiari 1 patients: A case series. Surg Neurol Int. 2013. 4: 6-
11. Lei ZW, Wu SQ, Zhang Z, Han Y, Wang JW, Li F. Clinical characteristics, imaging findings and surgical outcomes of Chiari malformation Type I in pediatric and adult patients. Curr Med Sci. 2018. 38: 289-95
12. Likert R. A technique for the measurement of attitudes. Arch Psychol. 1932. 22: 5-55
13. Odom GL, Finney W, Woodhall B. Cervical disk lesions. J Am Med Assoc. 1958. 166: 23-8
14. Tisell M, Wallskog J, Linde M. Long-term outcome after surgery for Chiari I malformation. Acta Neurol Scand. 2009. 120: 295-9
15. Tubbs RS, Beckman J, Naftel RP, Chern JJ, Wellons JC, Rozzelle CJ. Institutional experience with 500 cases of surgically treated pediatric Chiari malformation Type I. J Neurosurg Pediatr. 2011. 7: 248-56
16. Williams LE, Vannemreddy PS, Watson KS, Slavin KV. The need in dural graft suturing in Chiari I malformation decompression: A prospective, single-blind, randomized trial comparing sutured and sutureless duraplasty materials. Surg Neurol Int. 2013. 4: 26-
17. Yarbrough CK, Greenberg JK, Park TS. Clinical outcome measures in Chiari I malformation. Neurosurg Clin N Am. 2015. 26: 533-41