- Division of Neurological Surgery, Hospital das Clinicas HCFMUSP, University of Sao Paulo,
- School of Medicine, University of Sao Paulo,
- Service of Interventional Radiology, Radiology Institute, Hospital das Clinicas HCFMUSP, University of Sao Paulo, Sao Paulo, Brazil.
Correspondence Address:
João Paulo Mota Telles
Division of Neurological Surgery, Hospital das Clinicas HCFMUSP, University of Sao Paulo,
DOI:10.25259/SNI_443_2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Jefferson Rosi Júnior, João Paulo Mota Telles, Saul Almeida da Silva, Ricardo Ferrareto Iglesio, Mauricio Mandel Brigido, José Guilherme Mendes Pereira Caldas, Manoel Jacobsen Teixeira, Eberval Gadelha Figueiredo. Epidemiological analysis of 1404 patients with intracranial aneurysm followed in a single Brazilian institution. 20-Dec-2019;10:249
How to cite this URL: Jefferson Rosi Júnior, João Paulo Mota Telles, Saul Almeida da Silva, Ricardo Ferrareto Iglesio, Mauricio Mandel Brigido, José Guilherme Mendes Pereira Caldas, Manoel Jacobsen Teixeira, Eberval Gadelha Figueiredo. Epidemiological analysis of 1404 patients with intracranial aneurysm followed in a single Brazilian institution. 20-Dec-2019;10:249. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=9813
Abstract
Background: We sought to evaluate the epidemiology of intracranial aneurysms in relation to location, gender, age, presence of multiple aneurysms, and comorbidities in the Brazilian population.
Methods: We performed a prospective analysis of a cohort of 1404 patients diagnosed with intracranial aneurysm admitted to the Hospital das Clinicas of the University of Sao Paulo, a referral hospital for the treatment of cerebrovascular diseases in Brazil. Patients admitted between September 2009 and September 2018 with radiological diagnosis of intracranial aneurysm were included in the study.
Results: A total of 2251 aneurysms were diagnosed. Females accounted for 1090 aneurysms (77.6%) and the mean age at diagnosis was 54.9 years (ranging 15–88). The most common location was middle cerebral artery (MCA) with 593 aneurysms (26.3%) followed by anterior cerebral artery (ACA) with 417 aneurysms (18.5%) and internal carotid artery in the posterior communicating segment with 405 aneurysms (18.0%). Males had higher rates of ACA aneurysms (29.7%) while females had higher rates of MCA aneurysms (26.1%). Sorting by size, 492 aneurysms were 24 mm (5.2%). The occurrence of multiple aneurysms was associated with female gender (P P P = 0.121).
Conclusion: In this population, the occurrence of intracranial aneurysm is related to several factors, including gender, age, smoking, and hypertension. Our study brought to light important characteristics of a large number of Brazilian patients regarding epidemiology, location, size, and multiplicity of intracranial aneurysms.
Keywords: Epidemiology, Gender, Intracranial aneurysms, Risk factors, Smoking, Vascular disorders
INTRODUCTION
Intracranial aneurysms are the leading cause of spontaneous subarachnoid hemorrhage (SAH) accounting for up to 85% of cases.[
Several epidemiological studies evaluated the prevalence of aneurysms and the risk of rupture.[
In this study, we sought to analyze the epidemiology of intracranial aneurysms regarding location, gender, age, presence of multiple aneurysms, and comorbidities in the Brazilian population. These data might bring to light singular epidemiologic characteristics regarding intracranial aneurysm in the Brazilian and Latin American population.
PATIENTS AND METHODS
This study was carried out through a prospective analysis of a cohort of 1404 consecutive patients diagnosed with intracranial aneurysm admitted to the Hospital das Clinicas of the School of Medicine of the University of Sao Paulo, a referral hospital for the treatment of cerebrovascular diseases in Brazil. Data collection occurred between September 2009 and September 2018. Patients admitted to the emergency department with a history of SAH and ruptured aneurysm or patients evaluated at the outpatient clinic with unruptured intracranial aneurysms were included in the study. All patients underwent a digital subtraction angiography or a computed tomography angiography and only those with a confirmed diagnosis of intracranial aneurysm remained in the study.
Information on the location, size, and number of aneurysms were obtained by reports of neuroradiologists. The size used in the analysis corresponds to the biggest diameter of the aneurysm. Patients or family members reported the presence of comorbidities such as smoking and hypertension.
Data analysis was carried out using JASP 0.9.0.1 (2018, Amsterdam, The Netherlands). Student’s t-test was the standard test choice, but in most cases, Mann–Whitney U-test was the chosen test due to violation of equal variances assumptions evidenced by Levene’s test. Significance was established at P < 0.05.
RESULTS
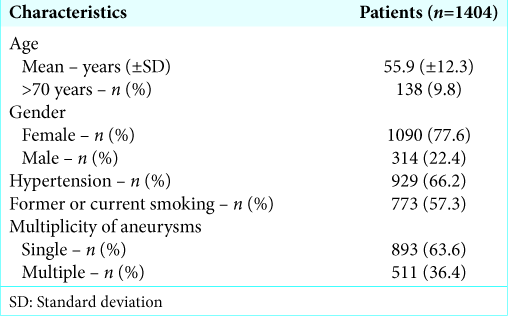
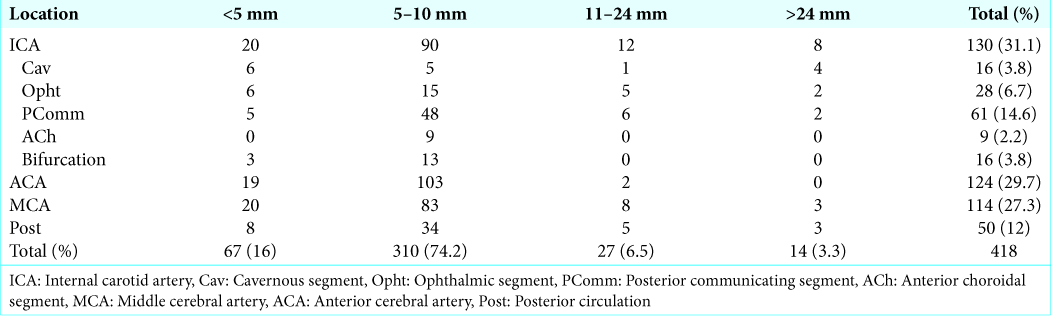
Patient characteristics are listed in
Location
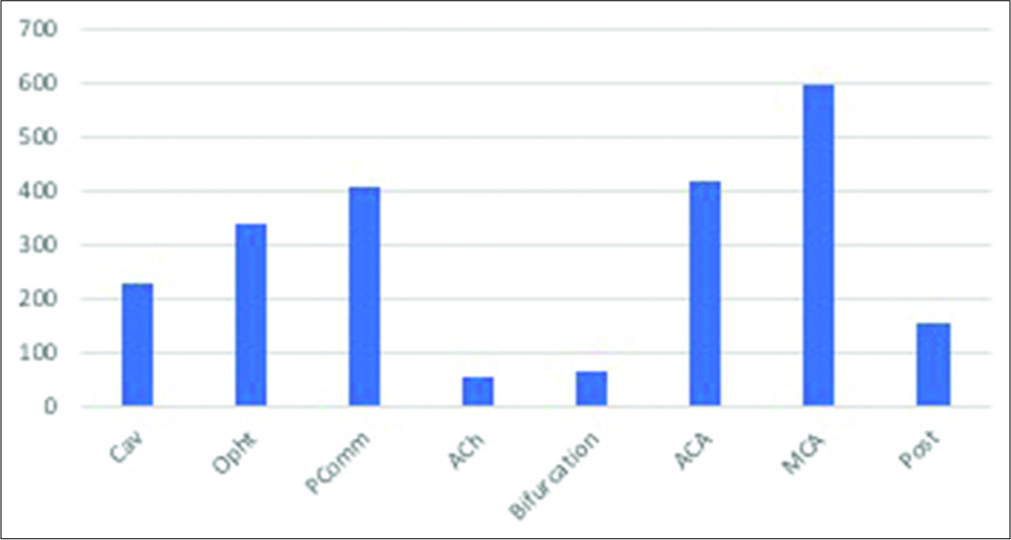
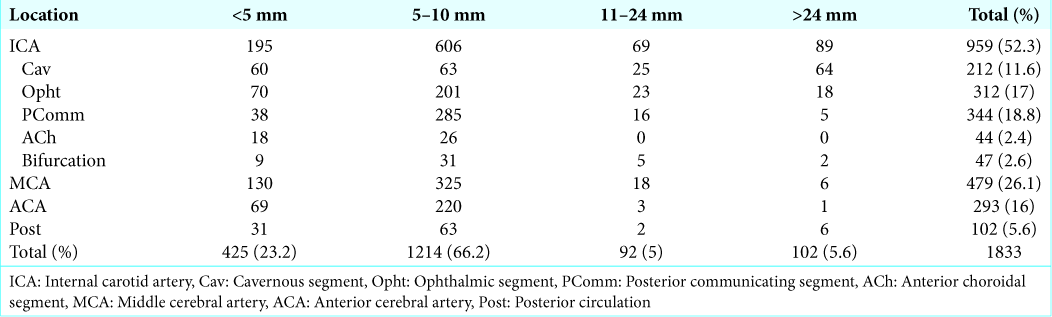
The most common location was middle cerebral artery (MCA) with 593 aneurysms (26.3%), followed by anterior cerebral artery (ACA) with 417 aneurysms (18.5%) and posterior communicating segment (PComm) of the internal carotid artery with 405 aneurysms (18%). Posterior circulation (Post) aneurysms accounted for only 6.7% (152) of all aneurysms in this study. All other locations are depicted in
Aneurysm location prevalence was different among males and females, as shown in
In sum, males were more prone to posterior circulation aneurysms when compared to females (10.2% vs. 3.4%), and this difference was statistically significant (χ2 = 21.400, P < 0.001). Neither hypertension (χ2 = 3.464, P = 0.18) nor smoking (χ2 = 4.801, P = 0.09) were significantly associated with location differences.
Multiple aneurysms
We diagnosed 2251 aneurysms, which yield a ratio of approximately 1.6 aneurysms per patient. Altogether, 511 (36.4%) patients had multiple aneurysms.
Female gender was associated with a higher prevalence of multiple aneurysms, with an average 1.682 against 1.331 aneurysms among men (W = 202,856.0, P < 0.001).
Smoking was also associated to higher number of multiple aneurysms (W = 219,393.0, P < 0.001), with smokers harboring an average 1.691 aneurysms against 1.496 for nonsmokers (W = 219,393.0, P < 0.001). Systemic arterial hypertension was not associated to multiple aneurysms (W = 211,110.5, P = 0.121, respectively).
DISCUSSION
We performed an epidemiological analysis of Brazilian patients diagnosed with cerebral aneurysms during almost a decade. In this study, we found 2251 aneurysms in 1404 patients with a 3.4 female/male ratio. Among the females, we found a higher incidence of MCA aneurysms. The preferential location of aneurysm in males was the ACA. The vast majority of the aneurysms was located in the anterior circulation and measured between 5 mm and 10 mm. About a third of the patients had more than one intracranial aneurysm.
To the best of our knowledge, this is the largest study involving Latin American patients diagnosed with intracranial aneurysms. Other studies have demonstrated the burden of the geographic location for the development and rupture of intracranial aneurysms.[
In our study, we found a higher rate of female patients with cerebral aneurysms (77.6%). This trending was also true in other studies performed worldwide. When analyzing studies that stratify the gender percentage in a series of patients with intracranial aneurysms,[
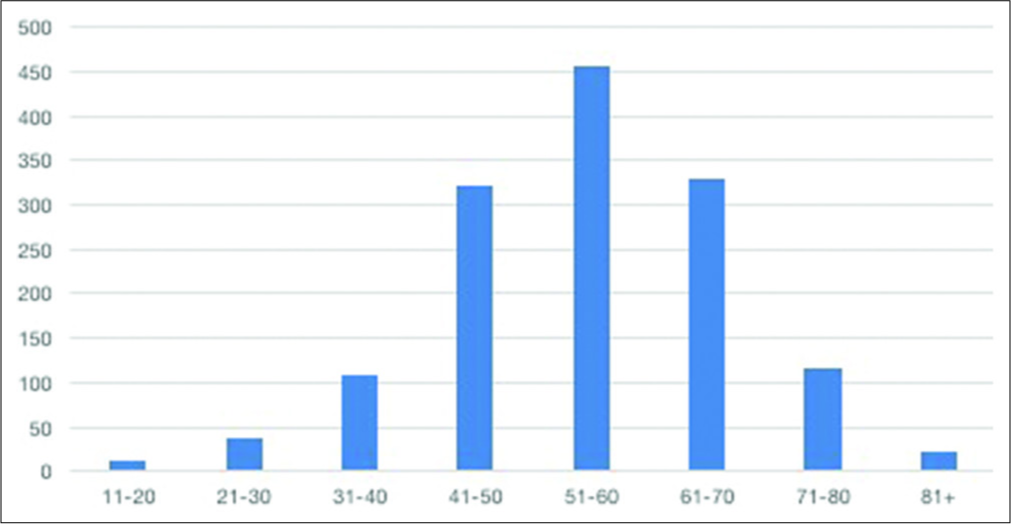
The mean age of the patients studied was 54.9 years, consistent with other studies that show an average age of 50 years for the world population diagnosed with intracranial aneurysm.[
Smoking and hypertension were reported in 57.3% and 66.2% of the patients, respectively. Both risk factor rates are significantly higher than nationwide or even state-based general statistics on smoking[
Aneurysms from the anterior circulation were far more common than vertebrobasilar aneurysms (93.2% vs. 6.7%) reproducing international findings.[
The reasons behind this distinctness remain subject of debate. Recent studies of computational fluid dynamics highlight the hemodynamics of blood flow as an important contributor to the development and rupture of intracranial aneurysms.[
Our results showed that most of the aneurysms found were between 5 mm and 10 mm in size. The previous studies have shown a higher rupture rate between aneurysms larger than 5 mm in size.[
Multiple aneurysms were found in 36.4% of patients, a slightly higher prevalence than the previous studies has shown.[
Data like these provide relevant data on the distribution of aneurysm’s prevalence worldwide, help to understand specific features of a given population, and delineate public health policies. Significant limitations of our study include being conducted in a single institution, although a referral center for cerebrovascular diseases. However, we encompass a large number of individuals and have provided important insights into cerebral aneurysm epidemiological characteristics for Brazilian and Latin American population.
CONCLUSION
The occurrence of intracranial aneurysm is related to several factors, including gender, age, smoking, hypertension, and geographical location. Our study brought to light important characteristics of a large number of Brazilian patients regarding epidemiology, location, size, and multiplicity of intracranial aneurysms. In addition, this paper fulfills a lack that existed when studying the prevalence of intracranial aneurysms in our continent.
References
1. Andrade SS, Stopa SR, Brito AS, Chueri PS, Szwarcwald CL, Malta DC. Self-reported hypertension prevalence in the Brazilian population: Analysis of the national health survey, 2013. Epidemiol Serv Saude. 2015. 24: 297-304
2. Brown RD, Broderick JP. Unruptured intracranial aneurysms: Epidemiology, natural history, management options, and familial screening. Lancet Neurol. 2014. 13: 393-404
3. Chalouhi N, Hoh BL, Hasan D. Review of cerebral aneurysm formation, growth, and rupture. Stroke. 2013. 44: 3613-22
4. Ellamushi HE, Grieve JP, Jäger HR, Kitchen ND. Risk factors for the formation of multiple intracranial aneurysms. J Neurosurg. 2001. 94: 728-32
5. Gasparotti R, Liserre R. Intracranial aneurysms. Eur Radiol. 2005. 15: 441-7
6. Ghods AJ, Lopes D, Chen M. Gender differences in cerebral aneurysm location. Front Neurol. 2012. 3: 78-
7. Greving JP, Wermer MJ, Brown RD, Morita A, Juvela S, Yonekura M. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: A pooled analysis of six prospective cohort studies. Lancet Neurol. 2014. 13: 59-66
8. Hamdan A, Barnes J, Mitchell P. Subarachnoid hemorrhage and the female sex: Analysis of risk factors, aneurysm characteristics, and outcomes. J Neurosurg. 2014. 121: 1367-73
9. Hughes JD, Bond KM, Mekary RA, Dewan MC, Rattani A, Baticulon R. Estimating the global incidence of aneurysmal subarachnoid hemorrhage: A systematic review for central nervous system vascular lesions and meta-analysis of ruptured aneurysms. World Neurosurg. 2018. 115: 430-70
10. Juvela S, Poussa K, Lehto H, Porras M. Natural history of unruptured intracranial aneurysms: A long-term follow-up study. Stroke. 2013. 44: 2414-21
11. Juvela S. Risk factors for multiple intracranial aneurysms. Stroke. 2000. 31: 392-7
12. Korja M, Lehto H, Juvela S. Lifelong rupture risk of intracranial aneurysms depends on risk factors: A prospective finnish cohort study. Stroke. 2014. 45: 1958-63
13. Liang L, Steinman DA, Brina O, Chnafa C, Cancelliere NM, Pereira VM. Towards the clinical utility of CFD for assessment of intracranial aneurysm rupture a systematic review and novel parameter-ranking tool. J Neurointerv Surg. 2019. 11: 153-8
14. Lindekleiv HM, Valen-Sendstad K, Morgan MK, Mardal KA, Faulder K, Magnus JH. Sex differences in intracranial arterial bifurcations. Gend Med. 2010. 7: 149-55
15. Malta DC, Vieira ML, Szwarcwald CL, Caixeta R, Brito SM, Dos Reis AA. Smoking trends among Brazilian population national household survey, 2008 and the national health survey, 2013. Rev Bras Epidemiol. 2015. 18: 45-56
16. Mhurchu CN, Anderson C, Jamrozik K, Hankey G, Dunbabin D. Hormonal factors and risk of aneurysmal subarachnoid hemorrhage: An international population-based, case-control study. Stroke. 2001. 32: 606-12
17. Nehls DG, Flom RA, Carter LP, Spetzler RF. Multiple intracranial aneurysms: Determining the site of rupture. J Neurosurg. 1985. 63: 342-8
18. Ostergaard JR, Høg E. Incidence of multiple intracranial aneurysms. Influence of arterial hypertension and gender. J Neurosurg. 1985. 63: 49-55
19. Park SK, Kim JM, Kim JH, Cheong JH, Bak KH, Kim CH. Aneurysmal subarachnoid hemorrhage in young adults: A gender comparison study. J Clin Neurosci. 2008. 15: 389-92
20. Rinne J, Hernesniemi J, Puranen M, Saari T. Multiple intracranial aneurysms in a defined population: Prospective angiographic and clinical study. Neurosurgery. 1994. 35: 803-8
21. UCAS Japan Investigators, Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012. 366: 2474-82
22. Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and meta-analysis. Lancet Neurol. 2011. 10: 626-36
23. Wardlaw JM, White PM. The detection and management of unruptured intracranial aneurysms. Brain. 2000. 123: 205-21
24. Wermer MJ, van der Schaaf IC, Algra A, Rinkel GJ. Risk of rupture of unruptured intracranial aneurysms in relation to patient and aneurysm characteristics: An updated meta-analysis. Stroke. 2007. 38: 1404-10
25. Wiebers DO, Whisnant JP, Huston J, Meissner I, Brown RD, Piepgras DG. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003. 362: 103-10
26. Zhao L, Zhang L, Zhang X, Li Z, Tian L, Wang YX. An analysis of 1256 cases of sporadic ruptured cerebral aneurysm in a single Chinese institution. PLoS One. 2014. 9: e85668-