- Departments of Neurosurgery, University of Alabama at Birmingham, Birmingham, Alabama.
- Departments of Endocrinology, University of Alabama at Birmingham, Birmingham, Alabama.
- Departments of Otolaryngology, University of Alabama at Birmingham, Birmingham, Alabama.
Correspondence Address:
Lauren E. Rotman
Departments of Neurosurgery, University of Alabama at Birmingham, Birmingham, Alabama.
DOI:10.25259/SNI_24_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Lauren E. Rotman, Elizabeth N. Alford, Matthew C. Davis, T. Brooks Vaughan, Bradford A. Woodworth, Kristen O. Riley. Preoperative radiographic and clinical factors associated with the visualization of intraoperative cerebrospinal fluid during endoscopic transsphenoidal resection of pituitary adenomas. 04-Apr-2020;11:59
How to cite this URL: Lauren E. Rotman, Elizabeth N. Alford, Matthew C. Davis, T. Brooks Vaughan, Bradford A. Woodworth, Kristen O. Riley. Preoperative radiographic and clinical factors associated with the visualization of intraoperative cerebrospinal fluid during endoscopic transsphenoidal resection of pituitary adenomas. 04-Apr-2020;11:59. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=9946
Abstract
Background: Intraoperative visualization of cerebrospinal fluid (CSF) during endoscopic endonasal resection of skull base tumors is the most common factor contributing to the development of postoperative CSF leaks. No previous studies have solely evaluated preoperative factors contributing to intraoperative CSF visualization. The purpose of this study was to identify preoperative factors predictive of intraoperative CSF visualization.
Methods: Retrospective review of patients who underwent transsphenoidal resection of pituitary adenomas was conducted. Clinical and radiographic variables were compared for those who had CSF visualized to those who did not. Nominal logistic regression models were built to determine predictive variables.
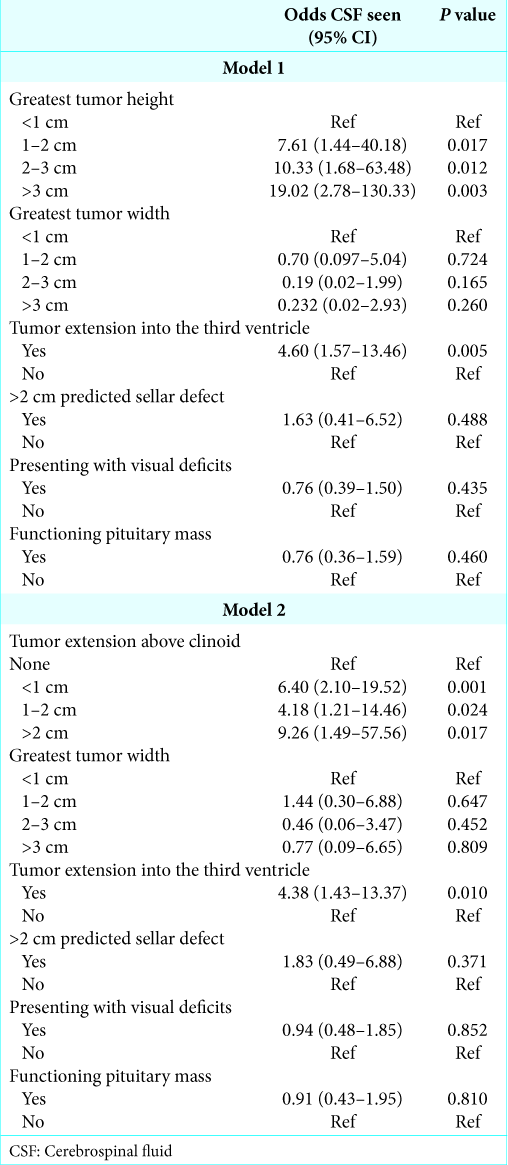
Results: Two hundred and sixty patients were included in the study. All significant demographic and radiographic variables on univariate analysis were included in multivariate analysis. Two multivariate models were built, as tumor height and supraclinoid extension were collinear. The first model, which considered tumor height, found that extension into the third ventricle carried a 4.60-fold greater risk of CSF visualization (P = 0.005). Increasing tumor height showed a stepwise, linear increase in risk; tumors >3 cm carried a 19.02-fold greater risk of CSF visualization (P = 0.003). The second model, which considered supraclinoid tumor extension, demonstrated that extension into the third ventricle carried a 4.38-fold increase in risk for CSF visualization (P = 0.010). Supraclinoid extension showed a stepwise, linear increase in intraoperative CSF risk; tumors with >2 cm of extension carried a 9.26-fold increase in risk (P = 0.017).
Conclusion: Our findings demonstrate that tumor height, extension into the third ventricle, and extension above the clinoids are predictive of intraoperative CSF visualization.
Keywords: Cerebrospinal fluid leak, Complications, Endoscopic transsphenoidal surgery, Pituitary adenoma, Skull base
INTRODUCTION
Advances in endoscopic skull base surgery have allowed for the resection of larger and more complex pathologies, leading to larger skull base defects, necessitating the development of complex skull base repair techniques.[
Postoperative CSF leak is the most common complication following endoscopic skull base surgery, with significant impact on patient morbidity and medical costs.[
While no gold standard protocol exists for management in the setting of visualized intraoperative CSF, most surgical teams favor complex skull base repair, typically through NSF.[
Despite its importance, few studies have endeavored to analyze this clinical question, and none have solely looked at preoperative predictors. In addition, major variations in tumor measurement methodologies between these previous studies have caused the relationship between tumor “size” and CSF visualization to remain poorly defined.[
The purpose of this study is to analyze preoperative clinical and radiographic variables to better understand their association with the risk of visualizing CSF intraoperatively. This information will be valuable to surgical teams for counseling patients and operative planning, as patients at higher risk for intraoperative CSF visualization are also more likely to require complex skull base repairs.
MATERIALS AND METHODS
Patient selection
All patients who underwent endoscopic transsphenoidal resection of pituitary adenoma between January 2009 and April 2019 were eligible for inclusion. Patients were excluded if there was no intraoperative documentation of the presence or absence of CSF visualization on review of surgical operative reports. This study was approved by the University of Alabama-Birmingham Institutional Review Board, protocol number 150610001, assurance number FWA00005960.
Preoperative and intraoperative workflow considerations
At our center, a neurosurgeon (KR) performs almost all pituitary procedures for the institution, and nearly, all cases are performed endoscopically. Surgical indications for tumor resection include apoplexy, endocrinopathy, and visual dysfunction. All cases not undergoing more extensive skull base repairs are repaired using a multilayer approach involving placement of an autologous fat graft in the intradural space, epidural overlay of a duraplasty made of porcine small intestine submucosa (Biodesign®, Cook Medical, Bloomington, IN), followed by bolstering with a synthetic high-density polyethylene implant (MedPor TSI, Medtronic). If preoperative MRI suggests a defect larger than 2 cm, NSF is considered, and patients are counseled accordingly. When an NSF is planned, the case is scheduled in coordination with an otolaryngologist (BW). Flap harvest is typically completed at the beginning of the case and placed in position on its conclusion. In cases where NSF is possible but not guaranteed, or in cases where NSF is not considered, neurosurgery proceeds with the approach and free graft repair is attempted. Otolaryngology is contacted for NSF repair in the setting of easy free graft dislodgement following a “tug test,” evidence of CSF leaking around the free graft during a Valsalva maneuver, and/or inability to adequately secure free graft material under a bony edge on at least three sides. High volume of CSF visualized intraoperatively and repeat surgeries is the setting in which unplanned rescue flaps are most frequently used.
Data collection
Electronic medical record review was conducted. Data were collected regarding patient demographics, presenting symptoms, prior operative history, tumor measurements, tumor pathologic characteristics, preoperative and postoperative endocrinological status, skull base repair technique, presence or absence of intraoperative visualized CSF, and presence of postoperative CSF leak. The presence of intraoperative CSF was determined from operative reports. Postoperative CSF leak was defined by persistent leakage of clear fluid from the nares spontaneously or with forward head tilt of 30°. Leaks were confirmed with patient clinical history and imaging findings. Confirmatory laboratory tests, such as beta-2 transferrin, were not commonly used as the results are not available in a timely manner so as to be useful in clinical decision-making. Cases were defined as patients having an intraoperative CSF visualized, while controls were subjects who did not have a visualized intraoperative CSF.
Tumor measurements
Qualitative and quantitative measures of tumor size were calculated for all patients. All measures were performed using contrasted MRI sequences. For patients unable to obtain an MRI, a CT was used. Independent statistical analysis was conducted for both MRI and CT measurements. No statistically significant difference was found between MRI and CT measurements and statistical analysis using both imaging modalities demonstrated nearly identical findings. As most patients are evaluated with MRI for pituitary tumors, only MRI data are presented in this study.
Qualitative descriptors included erosion (thinning) of the sella turcica without extension of tumor beyond sellar boundaries, tumor extension through the sella turcica into the sphenoid sinus, tumor extension into the third ventricle, and >2 cm predicted sellar defect as determined by radiographic tumor appearance/measured width. Intraoperative factors, such as tumor texture, were not considered, as the purpose of this study is to solely evaluate preoperative predictors. Quantitative measures of tumor size included maximal tumor height, maximal tumor width, and tumor extension above the anterior clinoids (all reported in mm).
Multivariate analysis of continuous variables often lacks the clinical utility to meaningfully guide clinical decision-making. When considering which patients to counsel for heightened risk of CSF leak and potential need for NSF, ease of use becomes important. Therefore, additional analyses were performed, subdividing continuous MRI radiographic variables into ordinal categories. Categories for tumor height and width included <1 cm, 1–2 cm, and >3 cm. Categories for extension above the anterior clinoid included no extension, <1 cm, 1–2 cm, and >2 cm.
Statistical analysis
All statistical analyses were conducted using JMP® 14 Statistical Software (SAS Institute, Cary, North Carolina, USA). Continuous variables are presented as mean ± standard deviation and were compared using t-tests. Categorical variables are presented as frequency and percentage and were compared using Chi-square tests. All variables that were found to significantly differ between groups were retained. Nominal logistic regression models were used to determine which the retained variables were predictive of intraoperative CSF egress. This manuscript was prepared using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist. P < 0.05 was considered statistically significant.
RESULTS
Patient demographics and clinical characteristics
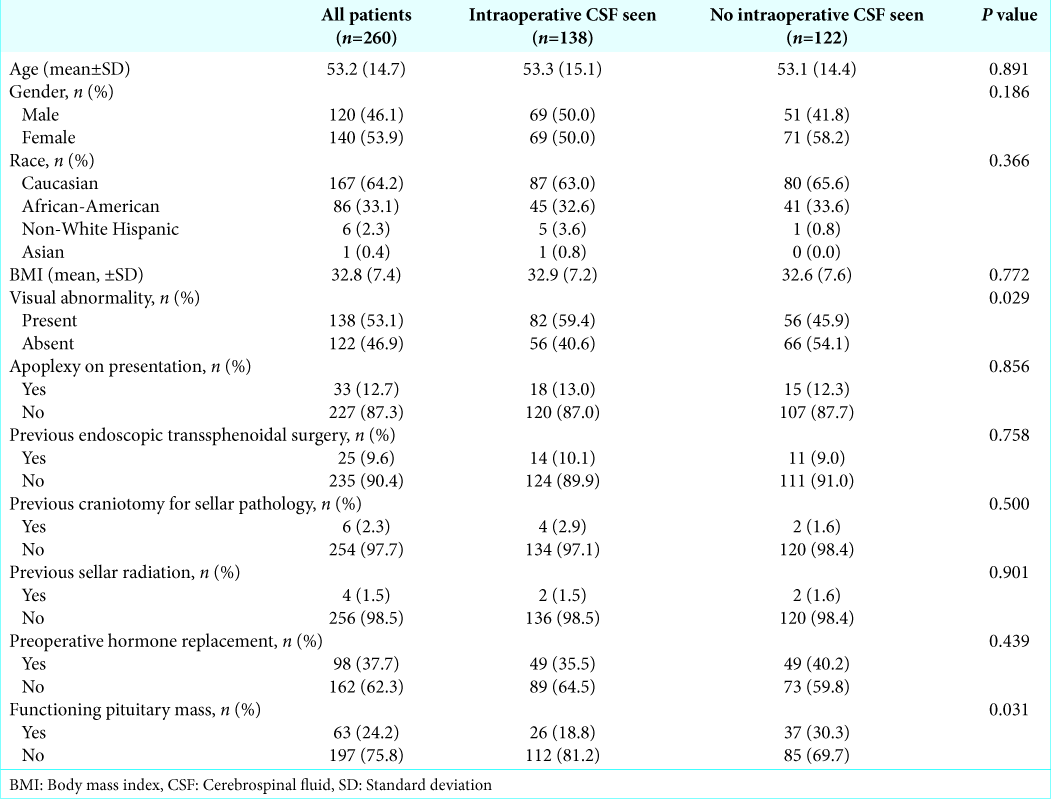
Two hundred and ninety-eight patients were screened for inclusion in this study. Thirty-eight patients were excluded due to lack of documentation of the presence or absence of CSF visualization on review of surgical operative reports. Two hundred and sixty patients were included in the final analysis, 138 (53.1%) were documented to have intraoperative CSF visualized, which is consistent with rates previously been documented in the literature. Average age at time of surgical resection was 53.2 years and 46.1% of patients were female. About 75.8% of tumors were nonfunctional, 53.1% of patients had visual abnormalities present on admission, 12.7% presented with pituitary apoplexy, and 9.6% had previously undergone transsphenoidal resection of a pituitary mass [
Operative management and postoperative characteristics
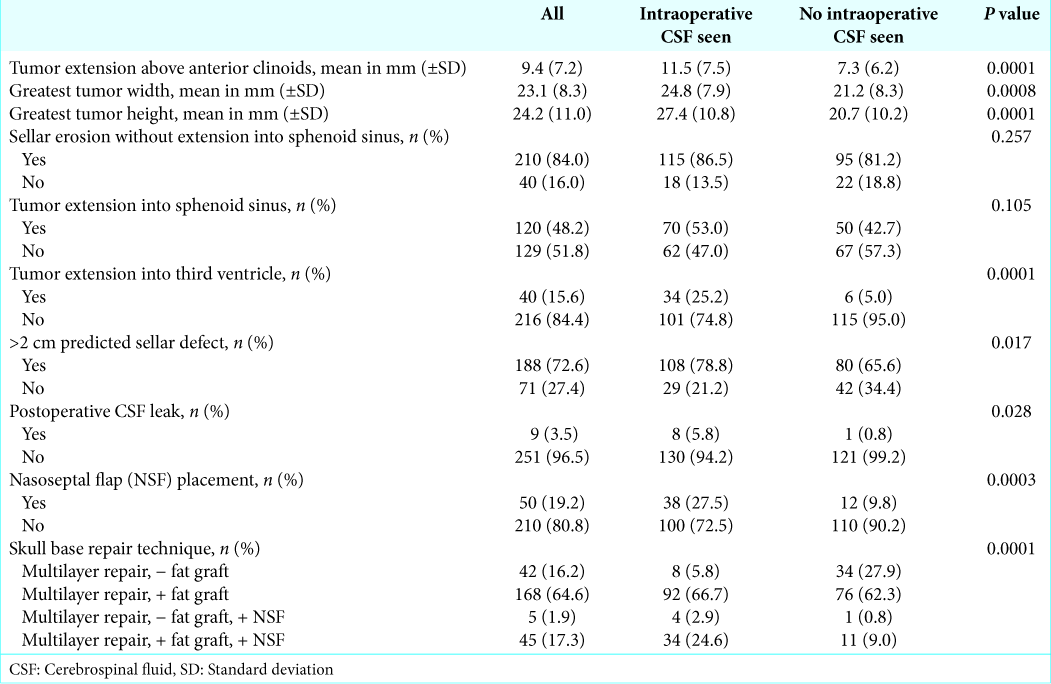
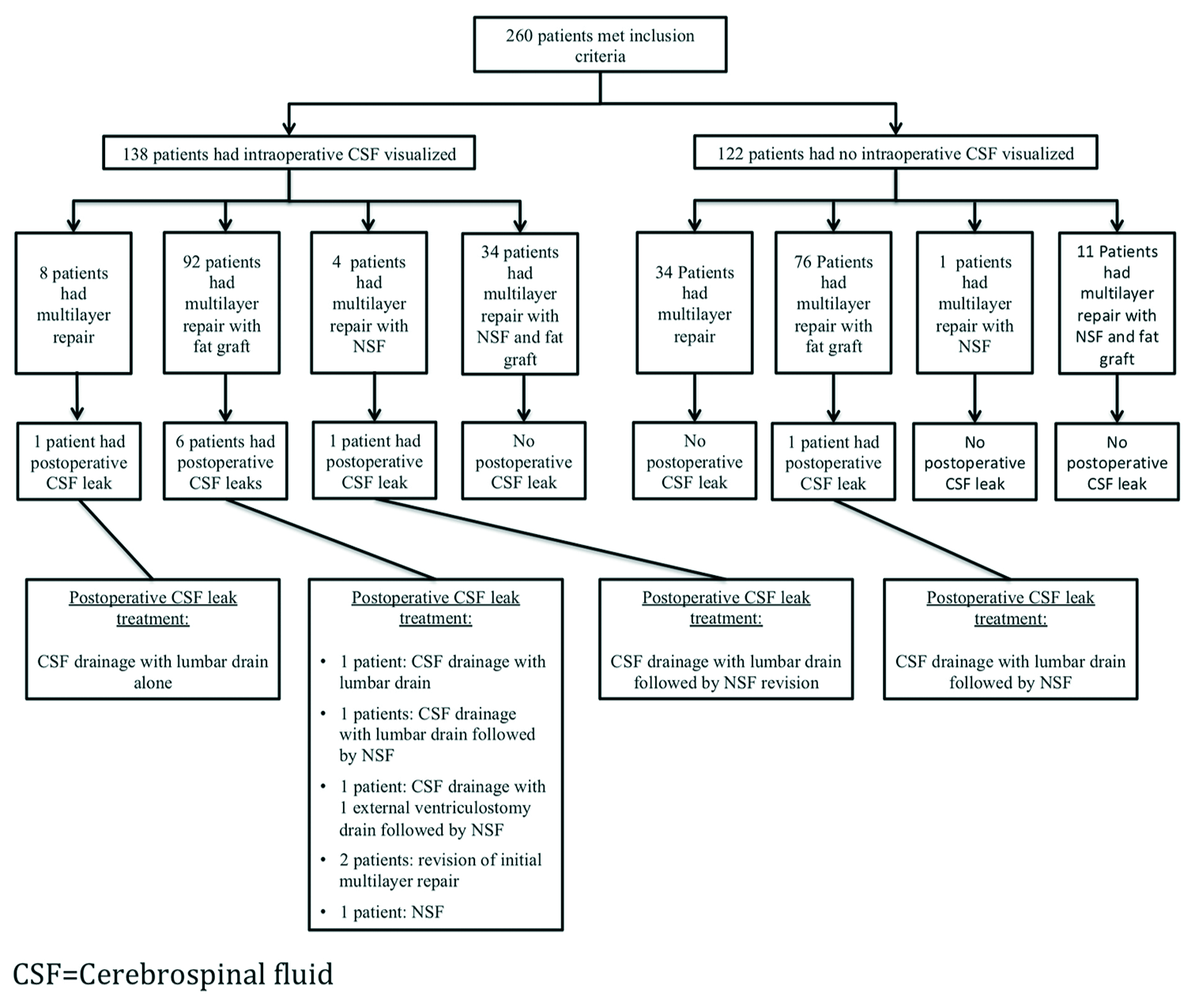
Of the patients with intraoperative CSF visualized, management included eight multilayer repairs, 92 multilayer repairs with autologous abdominal fat graft, four multilayer repairs with NSF, and 34 multilayer repairs with NSF and autologous abdominal fat graft (P < 0.0001,
Nine patients (3.5%) developed postoperative CSF leaks. The postoperative leak rate in those with visualized CSF intraoperatively was 5.8% as compared to 0.8% in those without the presence of an intraoperative CSF (P = 0.028,
Postoperative leak rates and repair strategies were not included in multivariate analysis as the purpose of this study is to analyze solely preoperative variables predictive of intraoperative CSF visualization with the goal of assisting surgical teams in determining which patients are at risk of requiring more complex skull base repairs. Including postoperative leak rates and intraoperative repairs would alter our results and detract from this study’s purpose.
Preoperative radiographic characteristics
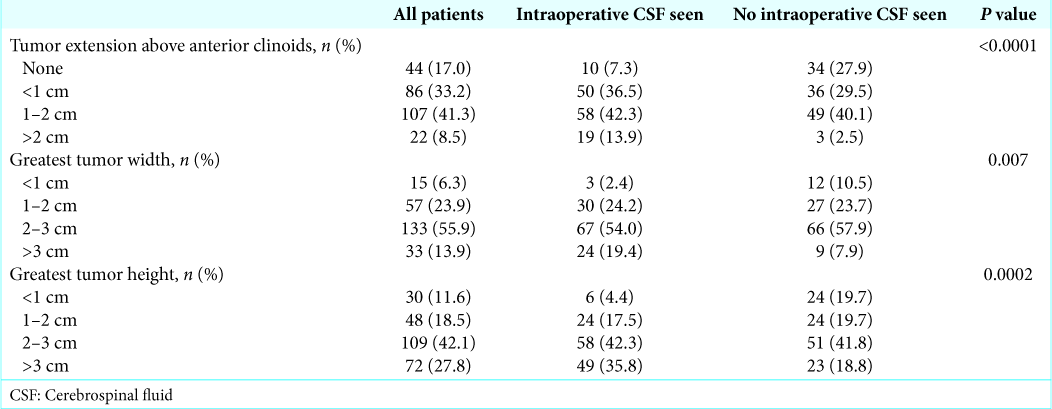
On review of preoperative imaging characteristics, patients with intraoperative CSF visualization were found to have larger tumor width (24.8 vs. 21.2 mm, P = 0.0008), tumor extension above the anterior clinoids (11.5 vs. 7.3 mm, P < 0.0001), and tumor height (27.4 vs. 20.7 mm, P < 0.0001) as compared to patient with no intraoperative CSF [
Bivariate analysis of variables when subdivided into ordinal categories
Continuous MRI radiographic variables were subdivided into ordinal categories to improve the ease of use and utility of the results in clinical decision-making. On bivariate analysis, intraoperative CSF visualization was significantly associated with tumor height (<1 cm, 1–2 cm, 2–3 cm, and >3 cm, P = 0.0002), tumor extension above the anterior clinoids (none, <1 cm, 1–2 cm, and >2 cm, P < 0.0001), and tumor width (<1 cm, 1–2 cm, 2–3 cm, and >3 cm, P = 0.007,
Nominal logistic regression modeling
Multivariate logistic regression models were constructed, consisting of the preoperative variables significantly associated with intraoperative CSF visualization on bivariate analysis. As tumor height and tumor extension above the anterior clinoids are collinear variables, two separate models were constructed to avoid data skewing. Model number one included all significant variables and tumor height, but did not include tumor extension above the clinoids. In this model, extension into the third ventricle carried a 4.60-fold greater risk of CSF visualization (P = 0.005), while increasing tumor heights showed a stepwise, linear increase in risk of CSF visualization. Relative to tumors <1 cm in size, tumors >3 cm carried a 19.02-fold greater risk of CSF visualization (P = 0.003,
The second model included all significant variables and extension above the clinoids, but did not include tumor height. In this model, tumor extension into the third ventricle carried a 4.38-fold greater risk of CSF visualization (P = 0.010), while supraclinoid extension showed a stepwise linear increase in risk of CSF visualization. Relative to tumors with no extension, tumors with >2 cm of extension carried a 9.26-fold increase in risk for CSF visualization (P = 0.017,
DISCUSSION
Demographic variables and intraoperative CSF visualization
In our study, postoperative CSF leak rates were significantly higher for those with intraoperative CSF visualized than those without, with an overall rate of CSF visualization during tumor resection of 53.1% and a postoperative CSF leak rate of 3.5%. This is reflective of what has been previously published in the literature, reconfirming the importance of intraoperative CSF visualization as a contributing factor in the development of postoperative CSF leaks.[
Of the demographic variables analyzed, only visual deficits on presentation and lack of tumor functionality were associated with intraoperative CSF visualization. On multivariate analysis, neither variable retained significance. The univariate relationship can be explained by the confounder of tumor size. Larger pituitary tumors are more likely to cause optic chiasm compression and subsequent visual field dysfunction.[
The previous studies have inconsistently reported an association between elevated BMI and intraoperative CSF visualization.[
Other studies have shown repeat surgery to be a risk factor for the visualization of intraoperative CSF.[
Radiographic characteristics and intraoperative CSF visualization
Differences in tumor measurement methodologies in previously published studies have caused the relationship between tumor size and the risk of visualizing CSF intraoperatively to remain poorly understood. Zhou et al. demonstrated significantly greater suprasellar extension and tumor “size” in cases with intraoperative CSF visualization; however, it is unclear whether “size” is referring to tumor width or height, and suprasellar extension does not appear to have been included in multivariate analyses.[
In our study, tumor size was described both quantitatively and qualitatively. It is not surprising that measures of the anteroposterior and left-right dimensions of the tumors, including >2 cm predicted sellar defect, tumor width, sellar erosion, and extrasellar extension into the sphenoid sinus, were not significantly associated with intraoperative CSF visualization on multivariate analysis. These variables are felt to predict the size of sellar defect and impact repair selection, but larger defects in the anteroposterior and left-right dimensions should not increase the risk of intraoperative CSF egress. Comparatively, variables that quantify the craniocaudal dimension of the tumors, including tumor height, intraventricular extension, and suprasellar extension above the anterior clinoid, were significantly associated with the visualization of intraoperative CSF. While the exact mechanism is not well understood, it has been hypothesized that tumors with greater craniocaudal extension develop incompetence of the diaphragma sellae secondary to sellar expansion, leading to exposed arachnoid that is at risk for thinning or developing defects, thereby increasing the risk of CSF egress.[
Management in the setting of visualized intraoperative CSF varies by institution. Conger et al. recently suggested a graded approach based on volume of intraoperative CSF egress with an overall postoperative CSF leak rate of 1.6% and a postoperative leak rate of 3.2% for patients with CSF visualized intraoperatively.[
Limitations
Due to the retrospective nature of this study, there is a potential for inaccuracy in data collection and reporting, as well as unintentional data entry errors. In our study, the visualization of intraoperative CSF was determined from operative reports, in which intraoperative findings are described, but no formal grading system or standardized method of reporting is used. It follows that formal grading of the amount of intraoperative CSF visualization, as previously described by Esposito et al., could not reliably be determined for our data.[
Despite these limitations, our findings yield key insights that build on our understanding of preoperative risk factors associated with intraoperative CSF visualization during endoscopic transsphenoidal resection of pituitary adenomas.
CONCLUSION
In this study, we found that tumor height, extension into the third ventricle, and extension above the clinoids are predictive of intraoperative CSF egress. Understanding risk factors for intraoperative CSF visualization helps predict which patients are more likely to require complex skull base repair, which can assist in counseling patients of the higher morbidity associated with complex repairs and can aid surgical teams in planning, as complex repairs are typically performed in a multidisciplinary fashion.
Declaration of patient consent
Patients consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alzhrani G, Sivakumar W, Park MS, Taussky P, Couldwell WT. Delayed complications after transsphenoidal surgery for pituitary adenomas. World Neurosurg. 2018. 109: 233-41
2. Black PM, Zervas NT, Candia GL. Incidence and management of complications of transsphenoidal operation for pituitary adenomas. Neurosurgery. 1987. 20: 920-4
3. Conger A, Zhao F, Wang X, Eisenberg A, Griffiths C, Esposito F. Evolution of the graded repair of CSF leaks and skull base defects in endonasal endoscopic tumor surgery: Trends in repair failure and meningitis rates in 509 patients. J Neurosurg. 2018. 130: 861-75
4. Dlouhy BJ, Madhavan K, Clinger JD, Reddy A, Dawson JD, O’Brien EK. Elevated body mass index and risk of postoperative CSF leak following transsphenoidal surgery. J Neurosurg. 2012. 116: 1311-7
5. Esposito F, Dusick JR, Fatemi N, Kelly DF. Graded repair of cranial base defects and cerebrospinal fluid leaks in transsphenoidal surgery. Oper Neurosurg (Hagerstown). 2007. 60: 295-303
6. Fraser S, Gardner PA, Koutourousiou M, Kubik M, Fernandez-Miranda JC, Snyderman CH. Risk factors associated with postoperative cerebrospinal fluid leak after endoscopic endonasal skull base surgery. J Neurosurg. 2018. 128: 1066-71
7. Hadad G, Bassagasteguy L, Carrau RL, Mataza JC, Kassam A, Snyderman CH. A novel reconstructive technique after endoscopic expanded endonasal approaches: Vascular pedicle nasoseptal flap. Laryngoscope. 2006. 116: 1882-6
8. Han ZL, He DS, Mao ZG, Wang HJ. Cerebrospinal fluid rhinorrhea following trans-sphenoidal pituitary macroadenoma surgery: Experience from 592 patients. Clin Neurol Neurosurg. 2008. 110: 570-9
9. Harvey RJ, Parmar P, Sacks R, Zanation AM. Endoscopic skull base reconstruction of large dural defects: A systematic review of published evidence. Laryngoscope. 2012. 122: 452-9
10. Hendricks BL, Shikary TA, Zimmer LA. Causes for 30-day readmission following transsphenoidal surgery. Otolaryngol Head Neck Surg. 2016. 154: 359-65
11. Jakimovski D, Bonci G, Attia M, Shao H, Hofstetter C, Tsiouris AJ. Incidence and significance of intraoperative cerebrospinal fluid leak in endoscopic pituitary surgery using intrathecal fluorescein. World Neurosurg. 2014. 82: e513-23
12. Karnezis TT, Baker AB, Soler ZM, Wise SK, Rereddy SK, Patel ZM. Factors impacting cerebrospinal fluid leak rates in endoscopic sellar surgery. Int Forum Allergy Rhinol. 2016. 6: 1117-25
13. Kuan EC, Yoo F, Patel PB, Su BM, Bergsneider M, Wang MB. An algorithm for sellar reconstruction following the endoscopic endonasal approach: A review of 300 consecutive cases. J Neurol Surg B Skull Base. 2018. 79: 177-83
14. Lund VJ, Stammberger H, Nicolai P, Castelnuovo P, Beal T, Beham A. European position paper on endoscopic management of tumours of the nose, paranasal sinuses and skull base. Rhinol Suppl. 2010. 22: 1-43
15. Magro E, Graillon T, Lassave J, Castinetti F, Boissonneau S, Tabouret E. Complications related to the endoscopic endonasal transsphenoidal approach for nonfunctioning pituitary macroadenomas in 300 consecutive patients. World Neurosurg. 2016. 89: 442-53
16. Mehta GU, Oldfield EH. Prevention of intraoperative cerebrospinal fluid leaks by lumbar cerebrospinal fluid drainage during surgery for pituitary macroadenomas. J Neurosurg. 2012. 116: 1299-303
17. Nayak JV, Rathor A, Grayson JW, Bravo DT, Velasquez N, Noel J. Porcine small intestine submucosal grafts improve remucosalization and progenitor cell recruitment to sites of upper airway tissue remodeling. Int Forum Allergy Rhinol. 2018. 8: 1162-8
18. Nishioka H, Haraoka J, Ikeda Y. Risk factors of cerebrospinal fluid rhinorrhea following transsphenoidal surgery. Acta Neurochir (Wien). 2005. 147: 1163-6
19. Pal A, Leaver L, Wass J. Pituitary adenomas. BMJ. 2019. 365: l2091-
20. Patel MR, Stadler ME, Snyderman CH, Carrau RL, Kassam AB, Germanwala AV. How to choose? Endoscopic skull base reconstructive options and limitations. Skull Base. 2010. 20: 397-404
21. Patel MR, Taylor RJ, Hackman TG, Germanwala AV, Sasaki-Adams D, Ewend MG. Beyond the nasoseptal flap: Outcomes and pearls with secondary flaps in endoscopic endonasal skull base reconstruction. Laryngoscope. 2014. 124: 846-52
22. Patel PN, Stafford AM, Patrinely JR, Smith DK, Turner JH, Russell PT. Risk factors for intraoperative and postoperative cerebrospinal fluid leaks in endoscopic transsphenoidal sellar surgery. Otolaryngol Head Neck Surg. 2018. 158: 952-60
23. Schmalisch K, Milian M, Schimitzek T, Lagrèze WA, Honegger J. Predictors for visual dysfunction in nonfunctioning pituitary adenomas implications for neurosurgical management. Clin Endocrinol (Oxf). 2012. 77: 728-34
24. Shiley SG, Limonadi F, Delashaw JB, Barnwell SL, Andersen PE, Hwang PH. Incidence, etiology, and management of cerebrospinal fluid leaks following trans-sphenoidal surgery. Laryngoscope. 2003. 113: 1283-8
25. Sigler AC, D’Anza B, Lobo BC, Woodard TD, Recinos PF, Sindwani R. Endoscopic skull base reconstruction: An evolution of materials and methods. Otolaryngol Clin North Am. 2017. 50: 643-53
26. Zhou Q, Yang Z, Wang X, Wang Z, Zhao C, Zhang S. Risk factors and management of intraoperative cerebrospinal fluid leaks in endoscopic treatment of pituitary adenoma: Analysis of 492 patients. World Neurosurg. 2017. 101: 390-5