- Department of Neurosurgery, Hospital Central Sur de Alta Especialidad PEMEX, Tlalpan, Ciudad de México, México.
- Department of Pathology, Hospital Central Sur de Alta Especialidad PEMEX, Tlalpan, Ciudad de México, México.
- Department of Neurosurgery, Hospital Central Norte PEMEX, Azcapotzalco, Ciudad de México, México.
Correspondence Address:
Rodolfo Pedro MolinaMartínez, Department of Neurosurgery, Hospital Central Sur de Alta Especialidad PEMEX, Tlalpan, Ciudad de México, México.
DOI:10.25259/SNI_1075_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Rodolfo Pedro Molina-Martínez1, Venus Damaris Medina-Illueca2, Carlos Betancourt-Quiroz1, Daniel Alejandro Vega-Moreno1, Andrés Alberto Moral-Naranjo1, Rosa María Vicuña-González2, Martha Leticia Llamas-Ceras2, Víctor Andrés Reyes-Rodríguez3, Rodrigo Efraín Hernández-Reséndiz3, Abraham Ibarra-de la Torre1, Ulises García-González1. 360-surgery for a giant cervical chordoma with involvement of the right vertebral artery. 08-Dec-2021;12:604
How to cite this URL: Rodolfo Pedro Molina-Martínez1, Venus Damaris Medina-Illueca2, Carlos Betancourt-Quiroz1, Daniel Alejandro Vega-Moreno1, Andrés Alberto Moral-Naranjo1, Rosa María Vicuña-González2, Martha Leticia Llamas-Ceras2, Víctor Andrés Reyes-Rodríguez3, Rodrigo Efraín Hernández-Reséndiz3, Abraham Ibarra-de la Torre1, Ulises García-González1. 360-surgery for a giant cervical chordoma with involvement of the right vertebral artery. 08-Dec-2021;12:604. Available from: https://surgicalneurologyint.com/surgicalint-articles/11269/
Abstract
Background: Chordomas are malignant tumors that arise from the remnants of the notochord. Complete en bloc radical resection with postoperative radiation therapy is currently considered the gold standard. Here, we performed a 360-staged approach to manage a C3-C4 chordoma that involved the right vertebral artery.
Case Description: A 40-year-old woman presented with a C3-C4 chordoma that invaded the right vertebral artery. She responded well to a circumferential approach including resection and stabilization.
Conclusion: A 40-year-old woman with a C3-C4 spinal chordoma was optimally managed with a combined anterior/posterior surgical approach including decompression/fusion.
Keywords: Chordoma, Cervical spine, Vertebral artery, 360-degrees
INTRODUCTION
Chordomas are malignant tumors that arise from the remnants of the notochord, anywhere in the axial skeleton.[
CASE REPORT
Presentation
A 40 YO woman presented with a 2-year history of the posterior neck and right shoulder pain; it worsened over the prior year.
Examination
On examination, she had 4+/5 mild weakness involving the right arm (C6, C7 distributions) along with hyperreflexia and decreased pinprick/hypoesthesia in the right C4-C6 distribution. Right Froment’s sign positive.
MR and CT findings
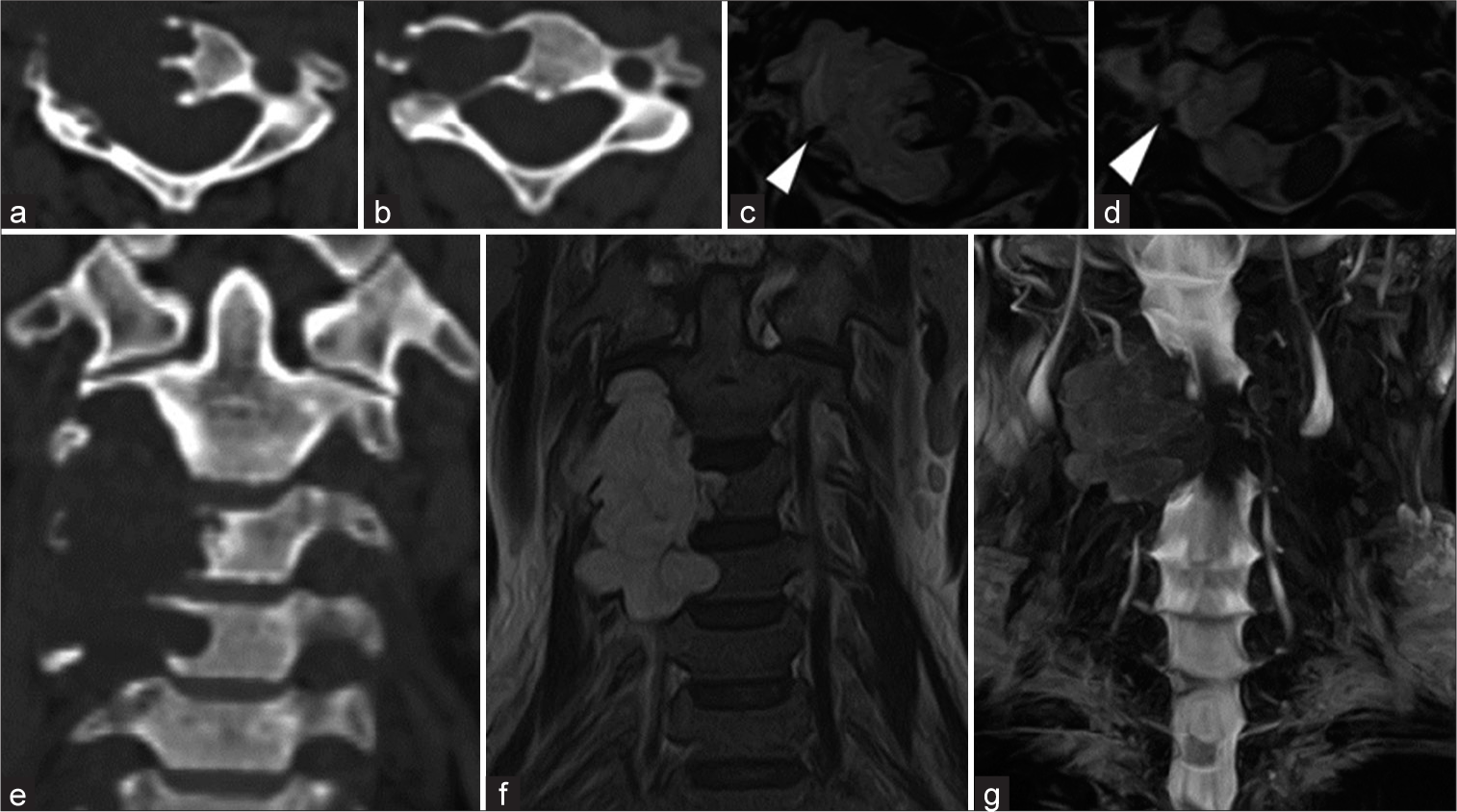
T2-weighted MR presented an isointense, homogeneous, well-defined lesion localized in the right side of the vertebral body of C3 and C4 invading the ipsilateral VA, myelo-scan presented compression of the spinal cord. Bone window CT demonstrated lytic lesion of the body and right transverse foramen of C3 and C4 [
Figure 1:
Pre-operative studies. (a) C3 CT bone window, (b) C4 CT bone window, (c) C3 T2-weighted MRI, right VA white arrow head, (d) C4 T2-weighted MRI, right VA white arrow head, E) cervical spine CT bone window, (f) cervical spine T2-weighted MRI, (g) cervical myelo-scan. CT: Computed tomography, VA: Vertebral artery, MR: Magnetic resonance.
Staged surgery
Part I
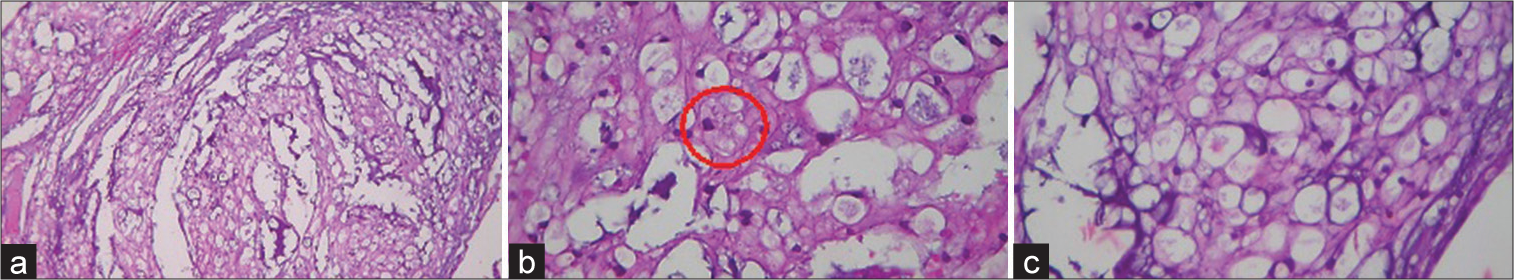
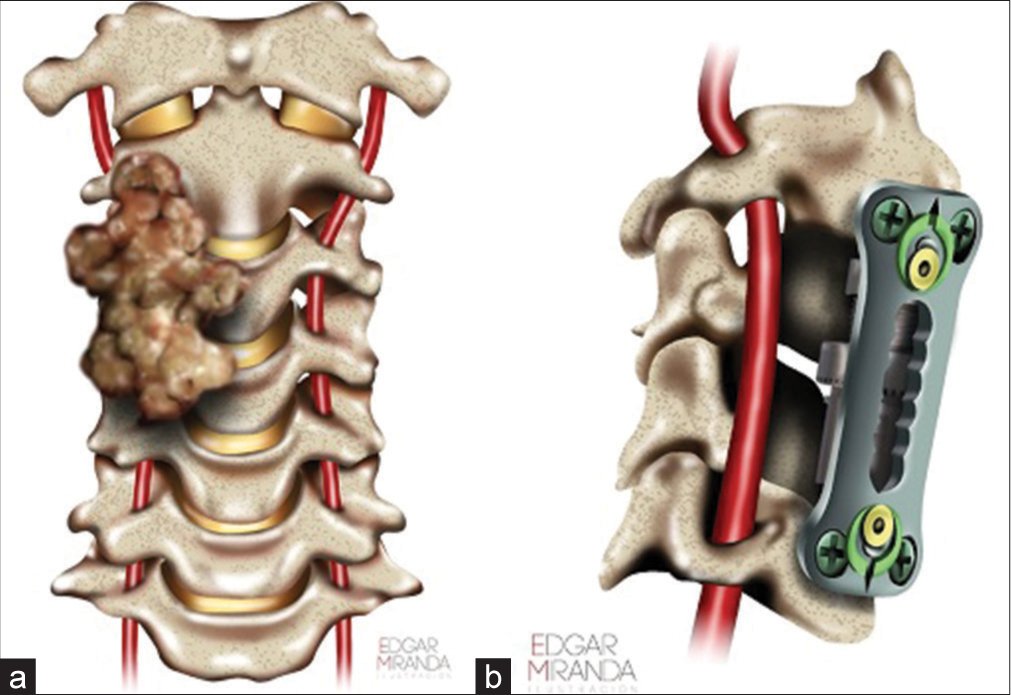
Stage I was a minimally invasive surgery (MIS) cervical laminectomy on the right of C3 and C4 (using tubular retractors and neurophysiology monitoring). The tumor was soft, gray-looking, with surrounding small vessels. With the help of an ultrasonic aspirator, the lesion was partially resected. There was an additional dorsal and lateral displacement of the right C5 root and 360° involvement of right VA. Only 50% of the lesion was resected and followed by routine closure. Pathology was consistent with a chordoma [
Figure 2:
Microscopic images. Hematoxylin and eosin stain. (a) Low power view (×5) Chondroid chordoma. Neoplastic epithelioid cells with eosinophilic to clear, bubbly cytoplasm arranged in nests, sheets, and individual in a myxoid stroma. (b) Hematoxylin and eosin stain. High power view (×40) Red circle demonstrates cells with vesicular chromatin and bubbly to vacuolated cytoplasm called physaliphorous cells. (c) Hematoxylin and eosin stain. High power view (×40) Physaliphorous cells reside in abundant myxoid stroma.
Part II
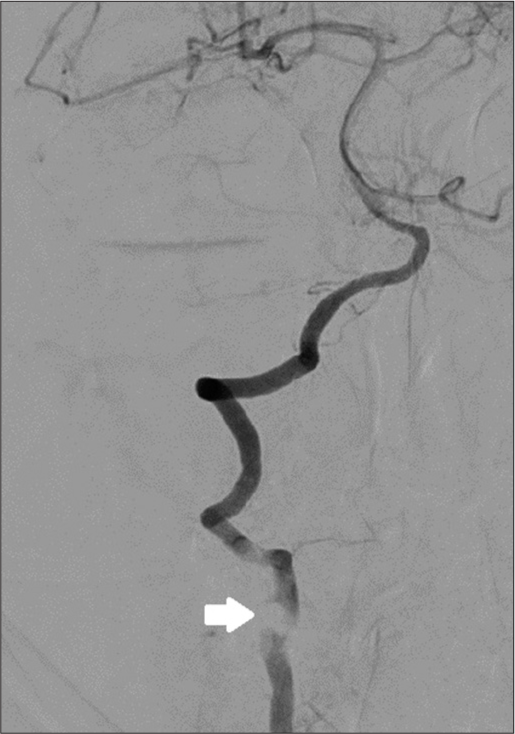
A preoperative angiogram was performed to evaluate the patency and dominance of both the compromised right VA and contralateral VA [
Part III
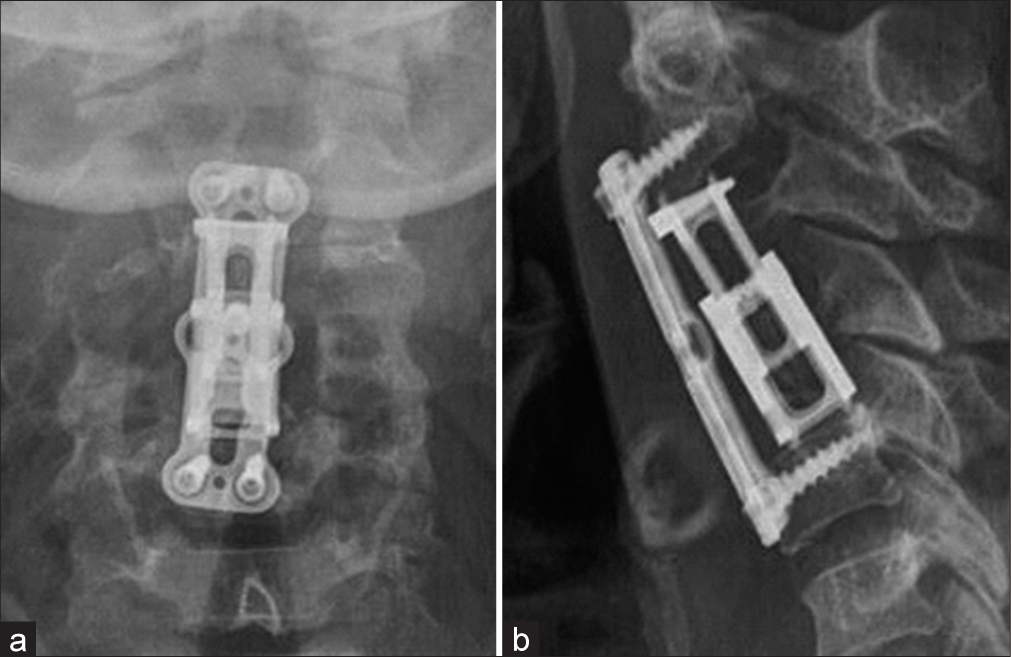
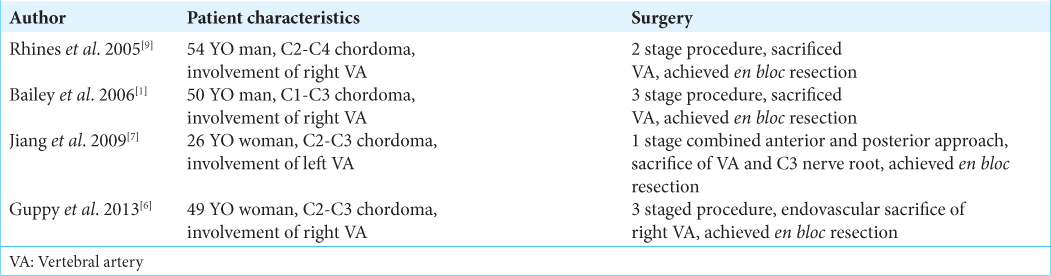
The patient was readmitted 4 weeks later for a 3rd stage procedure. A balloon test occlusion of the VA circulation was performed; the test was not tolerated due to right side VA dominance. The patient then underwent a posterior procedure aimed at complete radical resection of remaining posterior arch of C3 and C4 and tumor surrounding the right VA. A gross total removal was achieved under the operating microscope. The patient was routinely discharged 8 days later without pain, and no signs of cerebrospinal fluid leakage and/or new neurological dysfunction. Artist representation (
DISCUSSION
In our institution, we treated 13 patients with histologically confirmed diagnoses of chordoma in the last 10 years. Six patients were diagnosed with spinal chordomas. They presented at a mean age of 57.50 years. The average time from symptom onset to surgery was 5.7 months.
Cervical chordomas are not a common disease, they are often overlooked as a diagnostic possibility in patients with neck pain or palpable mass.[
The gold standard is en bloc resection, however, this was not initially considered in our patient due to the suspicion of right VA irruption, thus we had to perform a 360°, MIS, multiple staged approach to achieve maximal safe resection. For tumor extension, we used the Weinstein-Boriani-Biagini (WBB) classification.[
Currently, there are no guidelines regarding the management of cervical chordomas with invasion and encasement of the VA. In retrospect, a full angiography assessment of the patency of both VA prior to the first stage could have been made. After this case, we believe this step to be crucial in the management of this kind of pathology. Due to our own personal experience, we considered a MIS posterior approach to be the best option as the initial step, however an open full laminectomy of C3 and C4 is a suitable option as well, the decision should be made based on the surgeon expertise. Anterior tumor resection utilizing a C3, C4 corpectomy technique with an expandable cage and plating was effective for gross total anterior tumor resection. It additionally facilitated removal of tumor around the right VA.
There are limited reports of cervical chordomas managed with circumferential surgery where the VA was also involved, four cases are described [
CONCLUSION
Cervical chordomas are slow-growing malignant tumors resulting in spinal cord compression and occasional invasion of the VA. With appropriate preoperative testing, these lesions may be resected en bloc in stages, and accompanied by selective VA sacrifice.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bailey CS, Fisher CG, Boyd MC, Dvorak MFS. En bloc marginal excision of a multilevel cervical chordoma: Case report. J Neurosurg Spine. 2006. 4: 409-14
2. Boriani S, Weinstein JN, Biagini R. Primary bone tumors of the spine, terminology and surgical staging. Spine (Phila Pa 1976). 1997. 22: 1036-44
3. Chan P, Boriani S, Fourney DR, Biagini R, Dekutoski MB, Fehlings MG. An assessment of the reliability of the enneking and weinstein-boriani-biagini classifications for staging of primary spinal tumors by the spine oncology study group. Spine (Phila Pa 1976). 2009. 34: 384-91
4. Cui JF, Hao DP, Chen HS, Liu JH, Hou F, Xu WJ. Computed tomography and magnetic resonance imaging features of cervical chordoma. Oncol Lett. 2018. 16: 861-5
5. Denaro L, Berton A, Ciuffreda M, Loppini M, Candela V, Brandi ML. Surgical management of chordoma: A systematic review. J Spinal Cord Med. 2020. 43: 797-812
6. Guppy KH, Chakrabarti I, Isaacs RS, Jun JH. En bloc resection of a multilevel high-cervical chordoma involving C-2: New operative modalities: Technical note. J Neurosurg Spine. 2013. 19: 232-42
7. Jiang L, Liu ZJ, Liu XG, Ma QJ, Wei F, Lv Y. Upper cervical spine chordoma of C2-C3. Eur Spine J. 2009. 18: 293-300
8. Pamir MN, Al-Mefty O, Borba LA.editors. Chordomas: Technologies, Techniques, and Treatment Strategies. New York, United States: Thieme; 2017. p.
9. Rhines LD, Fourney DR, Siadati A, Suk I, Gokaslan ZL. En bloc resection of multilevel cervical chordoma with C-2 involvement. Case report and description of operative technique. J Neurosurg Spine. 2005. 2: 199-205
10. Wippold FJ, Koeller KK, Smirniotopoulos JG. Clinical and imaging features of cervical chordoma. Am J Roentgenol. 1999. 172: 1423-6