- Department of Neurosurgery, Kansai Medical University, Hirakata, Japan.
Correspondence Address:
Masahiro Nonaka, Department of Neurosurgery, Kansai Medical University, Hirakata, Japan.
DOI:10.25259/SNI_488_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Junichi Takeda, Masahiro Nonaka, Yi Li, Haruna Isozaki, Takamasa Kamei, Tetsuo Hashiba, Akio Asai. 5-Aminolevulinic acid fluorescence-guided endoscopic surgery for intraventricular tumors. 15-Jul-2022;13:302
How to cite this URL: Junichi Takeda, Masahiro Nonaka, Yi Li, Haruna Isozaki, Takamasa Kamei, Tetsuo Hashiba, Akio Asai. 5-Aminolevulinic acid fluorescence-guided endoscopic surgery for intraventricular tumors. 15-Jul-2022;13:302. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11721
Abstract
Background: In recent years, the efficacy of 5-aminolevulinic acid photodynamic diagnosis (5-ALA PDD) has been reported for various types of brain tumors, including malignant glioma. In addition, many reports have been published on the usefulness of neuroendoscopic surgery for intraventricular lesions. However, no systematic report is available on the combined use of 5-ALA PDD and neuroendoscopy for various intraventricular tumors.
Methods: We report 17 consecutive patients with intraventricular tumors. All patients received oral 5-ALA preoperatively and underwent endoscopic surgical treatment (resection or biopsy). We use a rigid endoscope with a built-in PDD system for intraoperative observation.
Results: Seven resections and 10 biopsies were performed. Histopathological diagnosis was confirmed in all 17 cases. Gross total resection was achieved in six of seven cases. The fluorescence positivity rates for each tumor were glioblastoma 100% (2/2), low-grade glioma 67% (2/3), subependymoma 0% (0/1), medulloblastoma 100% (1/1), pineoblastoma 0% (0/1), germ cell tumor 75% (3/4), diffuse large B-cell lymphoma 33% (1/3), and metastatic tumor 100% (2/2).
Conclusion: Our method has the potential to improve detection of residual tumors in blind spots and deep areas, as well as the accuracy and safety of biopsy procedures for intraventricular lesions that are difficult to view and treat under a microscope.
Keywords: 5-Aminolevulinic acid, Brain tumor, Intraventricular lesion, Neuroendoscope
INTRODUCTION
The efficacy of surgical treatment of malignant glioma following visualization with 5-aminolevulinic acid (5-ALA) has become widely known following the report by Stummer et al.[
Neuroendoscopic surgery can be used for removal and biopsy of deep-seated lesions as well as for performing endoscopic third ventriculostomy (ETV) simultaneously in cases of complicated hydrocephalus. Combined treatment with 5-ALA PDD and neuroendoscopy was first reported by Tamura et al. in 2007 for intraventricular glioma.[
MATERIALS AND METHODS
We included 17 consecutive patients with suspected malignant brain tumors located in the ventricles who underwent resection or biopsy at the Department of Neurosurgery, Kansai Medical University, between October 2014 and November 2021. All patients underwent neuroendoscopic surgery associated with 5-ALA PDD.
Surgical procedure
The patients were orally administered 20 mg/kg 5-ALA 3 h before induction of anesthesia. We used a special rigid endoscope (PDD endoscope system with D Light C system, KARL STORZ GmbH and Co., Tuttlingen, Germany) for intraoperative observation of 5-ALA fluorescence. The D Light C system has a 300 W Xe short-arc lamp and uses white light or blue light (380–420 nm) as excitation light. In addition, a dichroic long-pass filter is attached to the endoscope eyepiece for fluorescence observation. All fluorescence assessments were for in situ lesions in this study. The focus of the endoscope was set at a distance of 1 cm from the tip in the sheath before observing the lesion, which enabled the evaluation of fluorescence at the same distance from the lesion for all observations. The endoscope was used only at a tip angle of 0 degrees and the fluorescence was evaluated at the same angle by placing the lesion in the center of the field of view. For biopsies, the target and trajectory were determined with a neuronavigation system (Stealth Station or Stealth S7, Medtronic Navigation, Denver, CO.). A burr hole or small craniotomy was performed and a cortical incision of approximately 1 cm was performed. A transparent sheath (Neuroport Mini, Hakko Co., Japan) with a diameter of 6.8 mm was inserted into the target or ventricle and the neuroendoscope was inserted into the sheath to observe the fluorescence of the intraventricular lesion or the intraparenchymal lesion at the tip of the sheath. For resections, the navigation system was used and a small craniotomy was performed as the basic method, but in cases in which endoscopy was performed alone, the ViewSite Brain Access System (Vycor Medical, Inc., Boca Raton, FL, USA) was used, and forceps and suction tubes were used to remove the tumor in a piecemeal fashion. A brain retractor was used in cases in which the resection was performed using a combination of endoscopy and microscopy (OPMI Pentero, Carl Zeiss Surgical GmbH and Co., Oberkochen, Germany).
Postoperative evaluation
All patients underwent a computed tomography scan within 24 h postoperatively to assess the possibility of postoperative bleeding. For resections, magnetic resonance imaging (MRI) examinations were performed within 72 h postoperatively, and T1-weighted images (noncontrast and contrast) were used to evaluate the removal rate, which was classified as gross total resection (GTR) and partial resection. Histopathological diagnosis was performed for each patient and postoperative complications were evaluated.
Ethics committee approval and statement regarding patient consent
The protocol for this study was approved by the Ethics Committee of Kansai Medical University (No. 2018037). The requirement for written patient consent was waived because data were deidentified.
Statistical analysis
All statistical analyses were performed using JMP version 14.2 (SAS Institute, Cary, NC, USA). Fisher’s exact test was performed to compare the fluorescence evaluation between the following two groups: presence or absence of MRI contrast effect, intraventricular lesion or paraventricular lesion, and certain entity of tumor or not. A significant difference was considered to be P < 0.05.
RESULTS
The histopathological diagnosis was obtained in all 17 patients. Tumors were diagnosed as glioblastoma (GBM) in two cases, low-grade gliomas (LGGs) in three cases, subependymoma in one case, medulloblastoma in one case, pineoblastoma in one case, germ cell tumor in four cases, diffuse large B-cell lymphoma (DLBCL) in three cases, and metastatic tumor in two cases. Ten patients underwent biopsy and seven patients underwent resection. Of the seven resections, four cases were performed with endoscopy alone and GTR was achieved in 86% (6/7) of the cases. ETV was performed simultaneously with surgery in six cases (three biopsies and three resections). The clinical and histological characteristics of the patients, the details of the surgical procedures, and the fluorescent diagnoses are summarized in
Fluorescence properties of tumors
The fluorescence positivity rates by tumor were GBM 100% (2/2), LGGs 67% (2/3), subependymoma 0% (0/1), medulloblastoma 100% (1/1), pineoblastoma 0% (0/1), germ cell tumor 75% (3/4), DLBCL 33% (1/3), and metastatic tumor 100% (2/2).
Relationship between 5-ALA fluorescence positivity and tumor characteristics
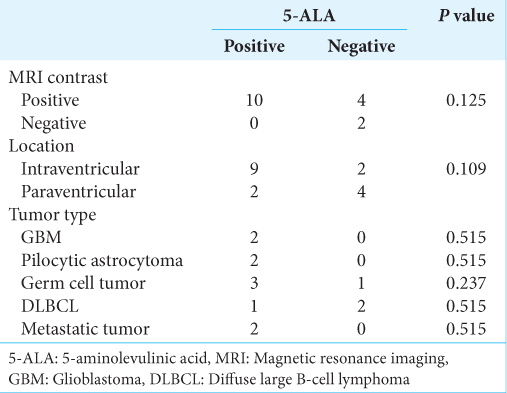
We performed statistical analysis of the relationship between 5-ALA positivity and MRI contrast enhancement (one patient was not given contrast medium due to renal dysfunction), intraventricular or paraventricular localization, and the histology of each tumor. No statistically significant correlations were found for any items, but relationships between 5-ALA positivity and MRI contrast enhancement (71%; 10/14), intraventricular localization (82%; 9/11), and histology of germ cell tumor (75%; 3/4) may exist [
Representative cases
Case No. 4: A biopsy was performed for a contrast-enhancing lesion located in the left caudate nucleus. We planned a contralateral transventricular approach. The lesion was approached from the right anterior horn and reached the contralateral anterior horn after performing a septostomy. 5-ALA PDD showed diffuse fluorescence at the wall of the ventricle presumably overlying the tumor, and we performed a biopsy of the same lesion and diagnosed it as DLBCL [
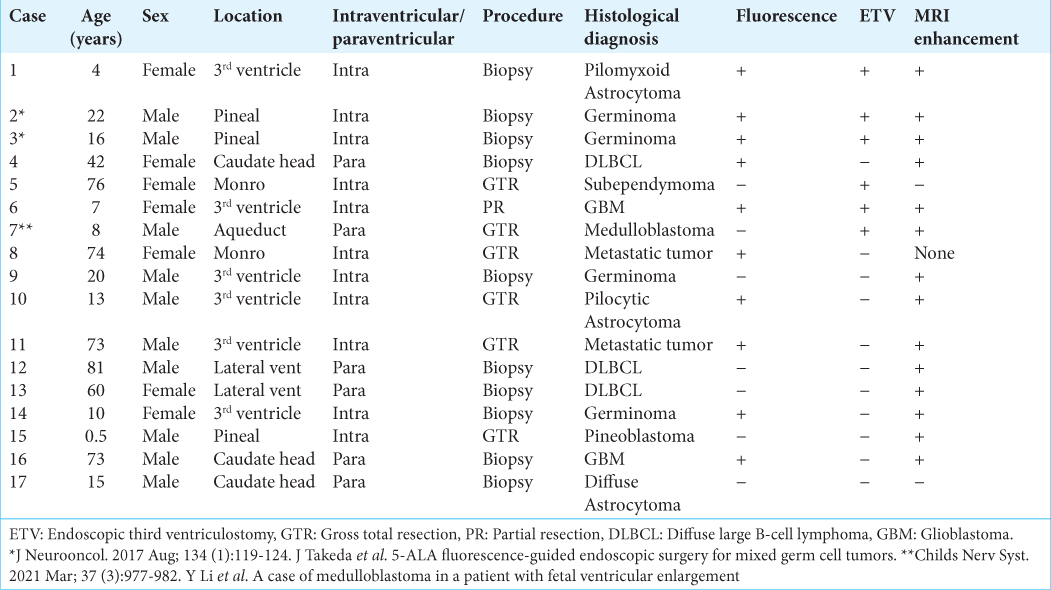
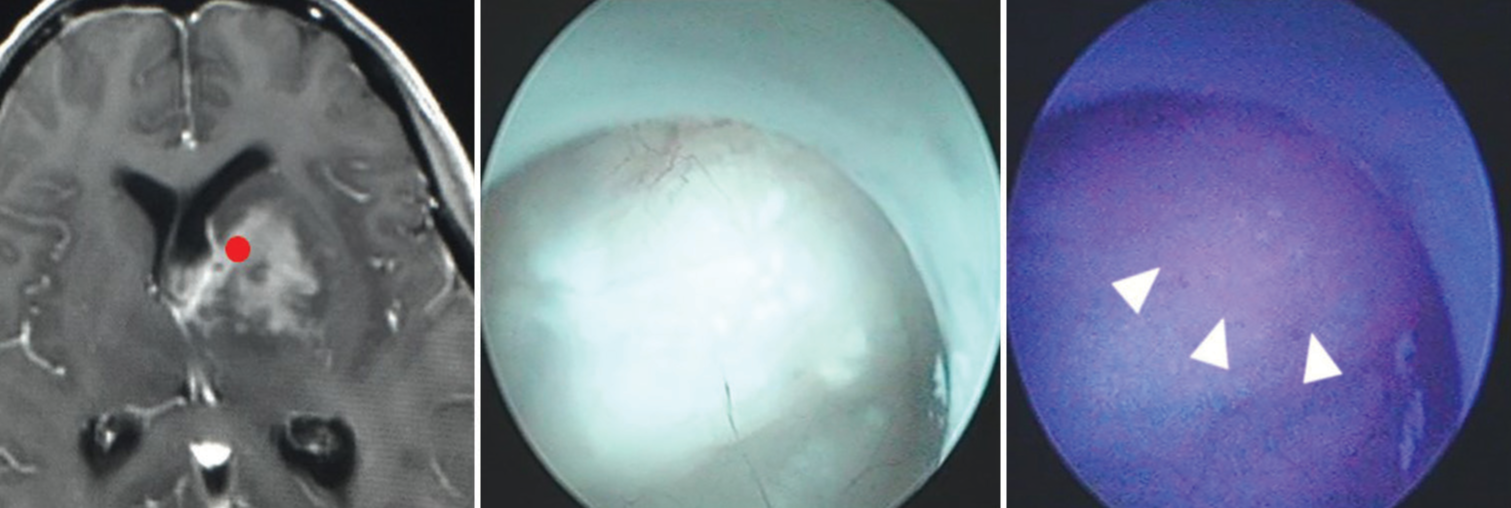
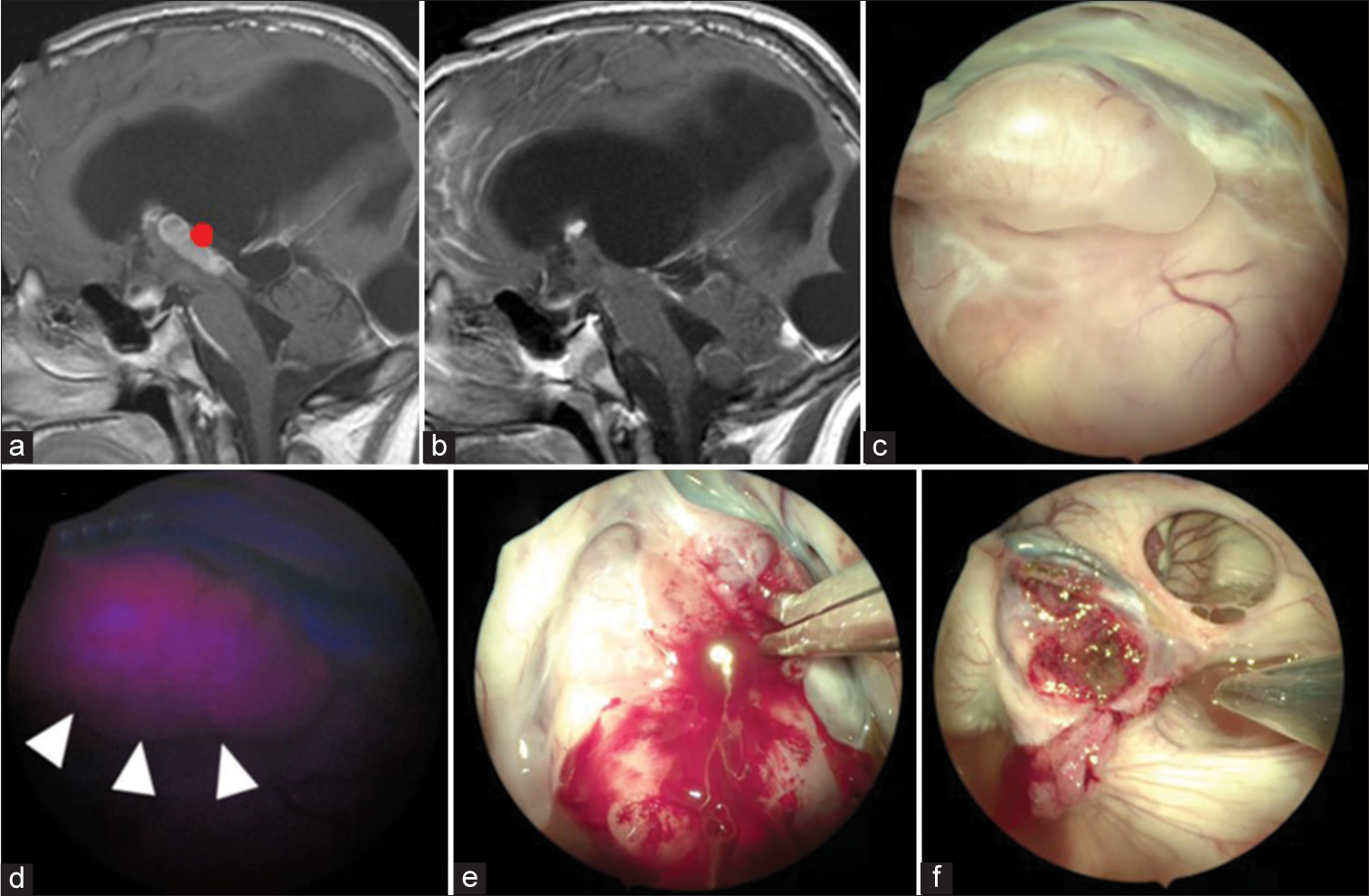
Case No. 10: A patient who had undergone a transventricular biopsy 10 years earlier at another hospital underwent resection of a pilocytic astrocytoma located in the third ventricle. A right anterior horn approach was performed through a small craniotomy and a 1.5 cm cortical incision. Because we were able to use the previous surgical corridor, we reached the right lateral ventricle directly with the endoscope without a sheath. PDD performed in the ventricles showed strong fluorescence in lesions consistent with tumors. Removal was performed in a piecemeal fashion using forceps and sometimes suction tubes, and hemostasis was confirmed under direct observation. The postoperative course was uneventful and GTR was confirmed by postoperative contrast-enhanced MRI [
Figure 2:
The patient in Case 10 underwent tumor resection for a third ventricle pilocytic astrocytoma through the right anterior horn. (a and b) MRI shows the presurgical view and postsurgical state of gross total removal. (c and d) Observation in the third ventricle by white light and blue light. Strong fluorescence was observed at the site of the tumor lesion. (e) Removal in a piecemeal manner was performed with forceps. (f) Gross total removal was achieved. Red dots indicate the site of observation. Arrowheads indicate the site of positive fluorescence.
DISCUSSION
Evaluation of 5-ALA fluorescence in intraventricular lesions
Intraventricular areas are rather difficult to reach during microscopic surgery and the possibility of endoscopic surgery has been discussed.[
Indications for 5-ALA
Recently, fluorescence positivity of 5-ALA has been reported in various types of tumors as well as malignant gliomas. Although off-label use of 5-ALA is expanding, its routine use is being questioned because it may not be useful in all tumors. Stummer et al. reported that it is useful for supratentorial and contrast-enhanced lesions in pediatric brain tumors.[
Advantages of our procedure
Since Tamura et al. reported the first 5-ALA PDD-guided endoscopic surgery for intraventricular glioma biopsy in 2007 as a technical note,[
Limitations and future
In some cases in our study, the ventricular wall was fluorescence positive despite the absence of an obvious tumor. We did not perform histopathological examination of those fluorescence-positive areas. Reporting on GBM, Hayashi et al. described the disruption of ependymal cells and the presence of tumor cells in fluorescence-positive areas of the ventricular wall.[
CONCLUSION
We report the results of 5-ALA PDD combined with neuroendoscopic surgery for various types of intraventricular tumors. We suggest that our procedure for intraventricular lesions may improve the detection of blind spots and deep-seated residual tumors, as well as the accuracy and safety of biopsies.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The author would like to thank Dr. Katsuyasu Koda for his helpful advice on statistical analysis.
References
1. Barbagallo GM, Certo F, Heiss K, Albanese V. 5-ALA fluorescence-assisted surgery in pediatric brain tumors: Report of three cases and review of the literature. Br J Neurosurg. 2014. 28: 750-4
2. Birski M, Furtak J, Krystkiewicz K, Birska J, Zielinska K, Sokal P. Endoscopic versus stereotactic biopsies of intracranial lesions involving the ventricles. Neurosurg Rev. 2021. 44: 1721-7
3. Boschi A, Della Puppa A. 5-ALA fluorescence on tumors different from malignant gliomas, Review of the literature and our experience. J Neurosurg Sci. 2019. 63: 661-9
4. Choo J, Takeuchi K, Nagata Y, Ohka F, Kishida Y, Watanabe T. Neuroendoscopic cylinder surgery and 5-aminolevulinic acid photodynamic diagnosis of deep-seated intracranial lesions. World Neurosurg. 2018. 116: e35-41
5. Chowdhry SA, Cohen AR. Intraventricular neuroendoscopy: Complication avoidance and management. World Neurosurg. 2013. 79: S15.e1-10
6. Cornelius JF, Kamp MA, Tortora A, Knipps J, Krause-Molle Z, Beez T. Surgery of small anterior skull base meningiomas by endoscopic 5-aminolevulinic acid fluorescence guidance: First clinical experience. World Neurosurg. 2019. 122: e890-5
7. Du B, Shan AJ, Peng YP, Wang J, Peng KW, Zhong XL. A new modified neuroendoscope technology to remove severe intraventricular haematoma. Brain Inj. 2018. 32: 1142-8
8. Eljamel MS, Leese G, Moseley H. Intraoperative optical identification of pituitary adenomas. J Neurooncol. 2009. 92: 417-21
9. Fiorindi A, Boaro A, Del Moro G, Longatti P. Fluorescein-guided neuroendoscopy for intraventricular lesions: A case series. Oper Neurosurg (Hagerstown). 2017. 13: 173-81
10. Fiorindi A, Longatti P. A restricted neuroendoscopic approach for pathological diagnosis of intraventricular and paraventricular tumours. Acta Neurochir (Wien). 2008. 150: 1235-9
11. García LM, Artero JM, Zamorano MB, Tejero IG. Fluorescence-guided resection with 5-aminolevulinic Acid of subependymomas of the fourth ventricle: Report of 2 cases: Technical case report. Neurosurgery. 2015. 11: E364-71
12. Giannetti AV, Alvarenga AY, de Lima TO, Pedrosa HA, Souweidane MM. Neuroendoscopic biopsy of brain lesions: Accuracy and complications. J Neurosurg. 2015. 122: 34-9
13. Hayashi Y, Nakada M, Tanaka S, Uchiyama N, Hayashi Y, Kita D. Implication of 5-aminolevulinic acid fluorescence of the ventricular wall for postoperative communicating hydrocephalus associated with cerebrospinal fluid dissemination in patients with glioblastoma multiforme: A report of 7 cases. J Neurosurg. 2010. 112: 1015-9
14. Inoue T, Endo T, Nagamatsu K, Watanabe M, Tominaga T. 5-aminolevulinic acid fluorescence-guided resection of intramedullary ependymoma: Report of 9 cases. Neurosurgery. 2013. 72: s159-68
15. Kamp MA, Grosser P, Felsberg J, Slotty PJ, Steiger HJ, Reifenberger G. 5-aminolevulinic acid (5-ALA)-induced fluorescence in intracerebral metastases: A retrospective study. Acta Neurochir (Wien). 2012. 154: 223-8
16. Marbacher S, Klinger E, Schwyzer L, Fischer I, Nevzati E, Diepers M. Use of fluorescence to guide resection or biopsy of primary brain tumors and brain metastases. Neurosurg Focus. 2014. 36: E10
17. Micko A, Rapoport BI, Youngerman BE, Fong RP, Kosty J, Brunswick A. Limited utility of 5-ALA optical fluorescence in endoscopic endonasal skull base surgery: A multicenter retrospective study. J Neurosurg. 2020. 30: 1-7
18. Millesi M, Kiesel B, Mischkulnig M, Martínez-Moreno M, Wöhrer A, Wolfsberger S. Analysis of the surgical benefits of 5-ALA-induced fluorescence in intracranial meningiomas: Experience in 204 meningiomas. J Neurosurg. 2016. 125: 1408-19
19. Moon JH, Kim SH, Shim JK, Roh TH, Sung KS, Lee JH. Histopathological implications of ventricle wall 5-aminolevulinic acid-induced fluorescence in the absence of tumor involvement on magnetic resonance images. Oncol Rep. 2016. 36: 837-44
20. Rapp M, Kamp M, Steiger HJ, Sabel M. Endoscopic-assisted visualization of 5-aminolevulinic acid-induced fluorescence in malignant glioma surgery: A technical note. World Neurosurg. 2014. 82: e277-9
21. Ritz R, Feigl GC, Schuhmann MU, Ehrhardt A, Danz S, Noell S. Use of 5-ALA fluorescence guided endoscopic biopsy of a deep-seated primary malignant brain tumor. J Neurosurg. 2011. 114: 1410-3
22. Roth J, Constantini S. 5ALA in pediatric brain tumors is not routinely beneficial. Childs Nerv Syst. 2017. 33: 787-92
23. Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006. 7: 392-401
24. Stummer W, Rodrigues F, Schucht P, Preuss M, Wiewrodt D, Nestler U. Predicting the “usefulness” of 5-ALA-derived tumor fluorescence for fluorescence-guided resections in pediatric brain tumors: A European survey. Acta Neurochir (Wien). 2014. 156: 2315-24
25. Stummer W, Stocker S, Wagner S, Stepp H, Fritsch C, Goetz C. Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery. 1998. 42: 518-25
26. Takeda J, Nonaka M, Li Y, Komori Y, Kamei T, Iwata R. 5-ALA fluorescence-guided endoscopic surgery for mixed germ cell tumors. J Neurooncol. 2017. 134: 119-24
27. Tamura Y, Kuroiwa T, Kajimoto Y, Miki Y, Miyatake S, Tsuji M. Endoscopic identification and biopsy sampling of an intraventricular malignant glioma using a 5-aminolevulinic acid-induced protoporphyrin IX fluorescence imaging system, Technical note. J Neurosurg. 2007. 106: 507-10
28. Tokairin K, Kazumata K, Gotoh S, Sugiyama T, Kobayashi H. Neuroendoscope-assisted aneurysm trapping for ruptured intraventricular aneurysms in moyamoya disease patients. World Neurosurg. 2020. 141: 278-83
29. Tsuzuki S, Aihara Y, Eguchi S, Amano K, Kawamata T, Okada Y. Application of indocyanine green (ICG) fluorescence for endoscopic biopsy of intraventricular tumors. Childs Nerv Syst. 2014. 30: 723-6
30. Yamamoto T, Ishikawa E, Miki S, Sakamoto N, Zaboronok A, Matsuda M. Photodynamic diagnosis using 5-aminolevulinic acid in 41 biopsies for primary central nervous system lymphoma. Photochem Photobiol. 2015. 91: 1452-7