- Departments of Neuroradiology, University of Florida College of Medicine, Jacksonville, Florida, United States.
- Departments of Neurosurgery, University of Florida College of Medicine, Jacksonville, Florida, United States.
- Departments of Pathology, University of Florida College of Medicine, Jacksonville, Florida, United States.
Correspondence Address:
Dinesh Rao
Departments of Neurosurgery, University of Florida College of Medicine, Jacksonville, Florida, United States.
DOI:10.25259/SNI_570_2019
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Dinesh Rao, Peter Fiester, Gazanfar Rahmathulla, Rafaat Makary, Daryoush Tavanaiepour. A case of a facial nerve venous malformation presenting with crocodile tear syndrome. 03-Jan-2020;11:3
How to cite this URL: Dinesh Rao, Peter Fiester, Gazanfar Rahmathulla, Rafaat Makary, Daryoush Tavanaiepour. A case of a facial nerve venous malformation presenting with crocodile tear syndrome. 03-Jan-2020;11:3. Available from: https://surgicalneurologyint.com/surgicalint-articles/9832/
Abstract
Background: Crocodile tears syndrome, also known as Bogorad syndrome, is characterized by lacrimation secondary to olfactory and gustatory stimuli and mastication. Crocodile tear syndrome is typically encountered as an uncommon complication of Bell’s palsy and usually occurs during the recovery phase of the disease course.
Case Description: We present a case of a 39-year-old male who presented with facial paralysis with ipsilateral crocodile tear syndrome caused by a slow flow venous malformation of the petrous bone and facial nerve.
Conclusion: We present a case of crocodile tear syndrome caused by a facial nerve venous malformation. To the best of our knowledge, this is the only case reported in literature.
Keywords: Bell’s palsy, Crocodile tear syndrome, Facial nerve venous malformation
INTRODUCTION
A 39-year-old male presented to the clinic with a complaint of the right hemifacial spasms that progressed to partial facial paralysis. The patient was diagnosed with Bell’s palsy and underwent Botox injection with improvement in the spasms. Several months later, he developed partial facial paralysis. The patient was unable to close his eyes and only had lacrimation from the right eye with eating and exercise. On presentation to the neurosurgical service, the patient had a House–Brackmann Grade 4 facial palsy which was noted to be moderate to severe with obvious weakness and disfiguring asymmetry. No other symptoms or relevant history was reported.
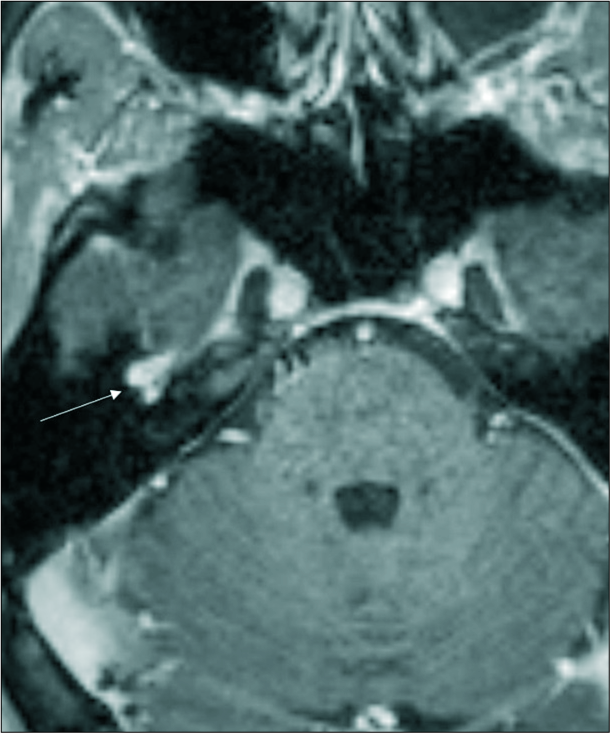
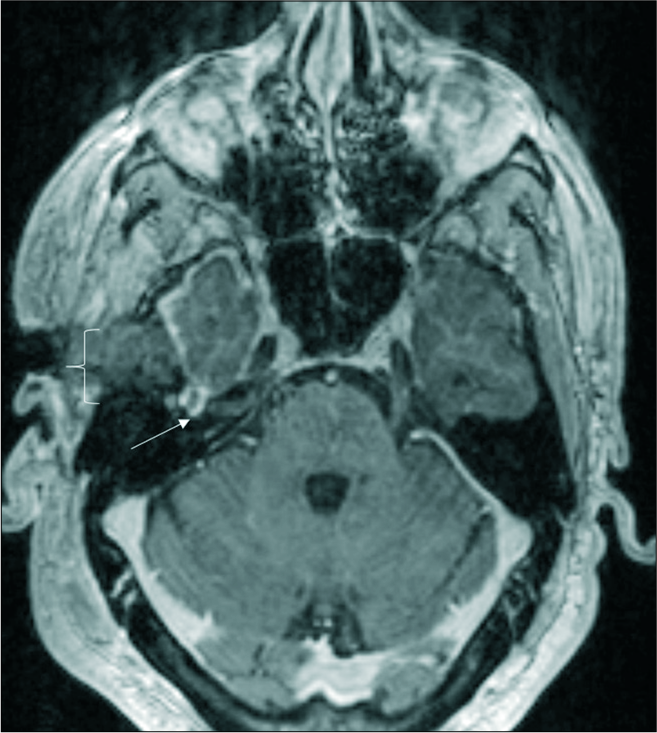
The patient underwent both magnetic resonance imaging (MRI) and computed tomography (CT) scan of the brain and temporal bones which demonstrated an enhancing mass lesion in the right petrous bone involving the geniculate ganglion (GG) of the right facial nerve [
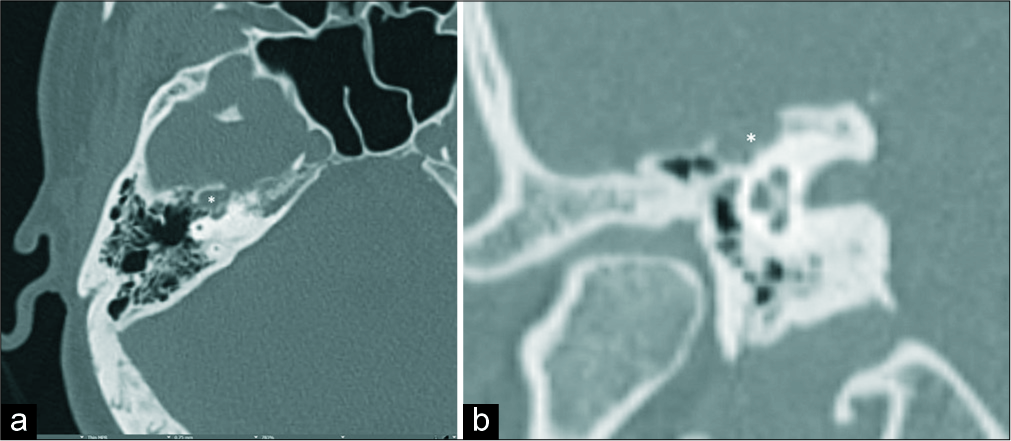
Figure 1:
(a) Axial unenhanced computed tomography (CT) demonstrates a lucent expansile lucent lesion in the right petrous bone involving the right facial geniculate segment (white asterisk), (b) magnified coronal unenhanced CT image demonstrating a lucent expansile lucent lesion in the right petrous bone involving the right facial geniculate segment (white asterisk).
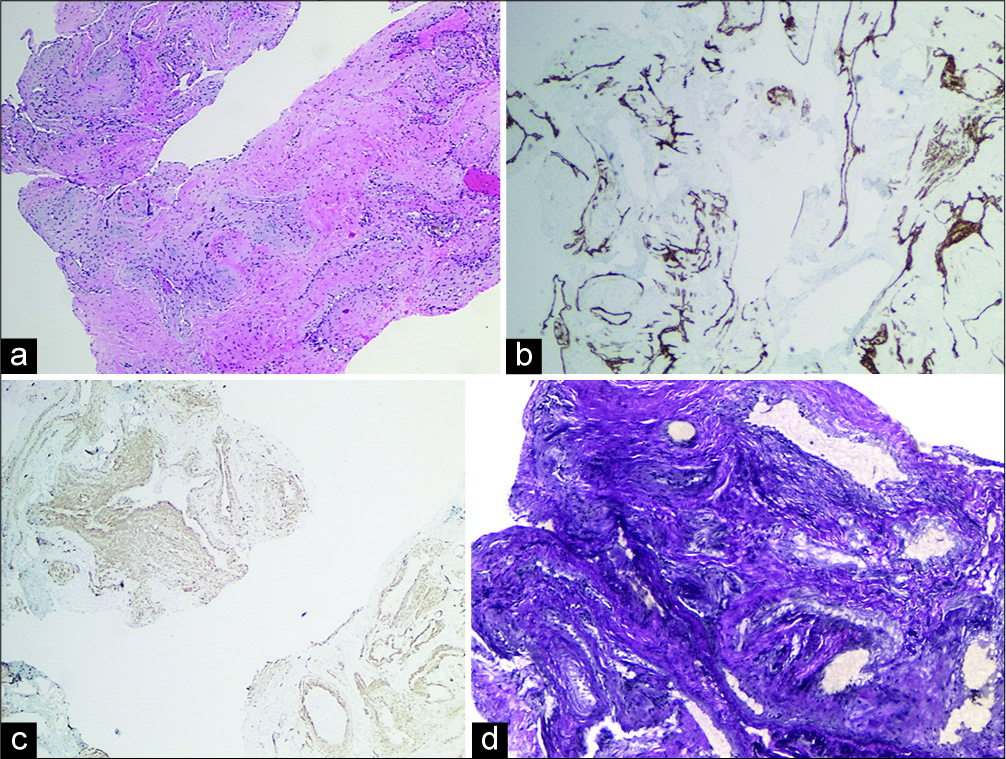
Pathologic examination of the lesion revealed a vascular malformation characterized by a conglomerate of blood vessels of variable caliber ranging from small to large [
Figure 4:
(a) Hematoxylin and eosin stain demonstrating a conglomerate of blood vessels ranging from small to large, (b) CD31 immunostain demonstrating the endothelium lining (brown), (c) smooth muscle actin (SMA) immunostain (SMA positive) demonstrating smooth muscle in the vessel walls, (d) elastin stain demonstrating the absence of elastic lamina, which is typical of venous malformation.
Immediately after resection, the patient regained some function and was able to lift his eyelid, but his facial palsy returned soon after surgery, with overall Grade 4 House–Brackmann palsy. Several months later, the patient underwent facial reanimation surgery and gold weight placement. He had good results postoperatively, but his facial palsy eventually returned. He was noted to have House–Brackmann Grade 4 facial palsy on follow-up appointments. The patient also reports residual symptoms related to crocodile tears syndrome (CTS).
DISCUSSION
Facial nerve venous malformations (FNVMs) are slow-growing, benign vascular lesions that arise from perineural capillary networks. They may involve any segment of the facial nerve and most commonly involve the GG.
FNVMs were previously described as hemangiomas and initially characterized on CT has have irregular ill-defined margins with intralesional bone spicules in a honeycomb pattern.[
On MRI, FNVMs are T1 isointense to hypointense, T2 hyperintense relative to adjacent gray matter and avidly enhance.[
FNVMs account for <1% of temporal bone lesions and 18% of facial nerve tumors.[
To the best of our knowledge, parasympathetic symptoms related to facial nerve injury have not been reported with FNMV.
CTS, also known as Bogorad syndrome, is characterized by lacrimation caused by olfactory and gustatory stimuli and mastication. The phenomenon is also known as paroxysmal lacrimation or the gustolacrimal reflex.[
During the recovery from Bell’s palsy, the parasympathetic fibers undergo regeneration but are misdirected, with fibers previously destined for salivary glands growing to the ipsilateral lacrimal gland. The regenerating nerves grow along the greater superficial petrosal nerve (GSPN) which results in olfactory or gustatory stimulation causing ipsilateral lacrimation.[
The incidence of Bell’s palsy is 0.08% per year and increases with age.[
Treatment
The most commonly accepted form of the treatment of FNVM is surgical excision.[
Regarding the clinical management of CTS, different treatment options have been used to stop lacrimation including guanethidine to block adrenergic receptors, propantheline bromide, and homatropine hydrobromide drops; however, the side effects have caused them to fall out of use.[
Surgical management, including excision of the palpebral lobe of the lacrimal gland, neurolysis of the chorda tympani or vidian nerve, and sphenopalatine nerve block, have been suggested to treat CTS but may be ineffective due to redundant innervation and come with significant morbidity such as vision loss and loss of taste.
The most commonly used treatment of CTS is Botox injection of the lacrimal gland. Botox is an acetylcholine esterase inhibitor and stops transmission along the aberrant parasympathetic nerves to the affected lacrimal gland.[
Differential diagnosis
The most common imaging differential diagnosis for an FNVM is a schwannoma. Schwannomas more commonly occur at the internal auditory canal (IAC) and present with hearing loss. Hemangiomas occurring at the GG or IAC are typically smaller and more symptomatic.[
CONCLUSION
We report a case of CTS in a 39-year-old male who presented with facial paralysis related to a venous malformation involving the GG of the right facial nerve. To the best of our knowledge, this is the first case of CTS reported in literature related to a vascular malformation. The diagnosis of CTS is usually made 6–9 months after the onset of Bell’s palsy and is characterized by lacrimation occurring with olfactory and gustatory stimulation and mastication. The most common treatment option is the injection of Botox directly into the lacrimal gland with relief of symptoms lasting approximately 6 months.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Achilli V, Mignosi S. Facial nerve hemangioma. Otol Neurotol. 2002. 23: 1003-4
2. Benoit MM, North PE, McKenna MJ, Mihm MC, Johnson MM, Cunningham MJ. Facial nerve hemangiomas: Vascular tumors or malformations?. Otolaryngol Head Neck Surg. 2010. 142: 108-14
3. Bogorad FA. The symptom of crocodile tears. F. A. Bogorad. Introduction and translation by austin seckersen. J Hist Med Allied Sci. 1979. 34: 74-9
4. Friedman O, Neff BA, Willcox TO, Kenyon LC, Sataloff RT. Temporal bone hemangiomas involving the facial nerve. Otol Neurotol. 2002. 23: 760-6
5. Glasscock ME, Smith PG, Schwaber MK, Nissen AJ. Clinical aspects of osseous hemangiomas of the skull base. Laryngoscope. 1984. 94: 869-73
6. Guerin JB, Takahashi EA, Lane JI, Hoxworth JM, Weindling SM, Blessing MM. Facial nerve venous malformation: A radiologic and histopathologic review of 11 cases. Laryngoscope Investig Otolaryngol. 2019. 4: 347-52
7. Lahlou G, Nguyen Y, Russo FY, Ferrary E, Sterkers O, Bernardeschi D. Geniculate ganglion tumors: Clinical presentation and surgical results. Otolaryngol Head Neck Surg. 2016. 155: 850-5
8. Lo WW, Horn KL, Carberry JN, Solti-Bohman LG, Wade CT, Brackmann DD. Intratemporal vascular tumors: Evaluation with CT. Radiology. 1986. 159: 181-5
9. Mangham CA, Carberry JN, Brackmann DE. Management of intratemporal vascular tumors. Laryngoscope. 1981. 91: 867-76
10. McCoy FJ, Goodman RC. The crocodile tear syndrome. Plast Reconstr Surg. 1979. 63: 58-62
11. Mijangos SV, Meltzer DE. Case 171: Facial nerve hemangioma. Radiology. 2011. 260: 296-301
12. Modi P, Arsiwalla T.editorsCrocodile Tears Syndrome. Statpearls: NCBI Bookshelf; 2018. p.
13. Montoya FJ, Riddell CE, Caesar R, Hague S. Treatment of gustatory hyperlacrimation (crocodile tears) with injection of botulinum toxin into the lacrimal gland. Eye (Lond). 2002. 16: 705-9
14. Nava-Castañeda A, Tovilla-Canales JL, Boullosa V, Tovilla-y-Pomar JL, Monroy-Serrano MH, Tapia-Guerra V. Duration of botulinum toxin effect in the treatment of crocodile tears. Ophthalmic Plast Reconstr Surg. 2006. 22: 453-6
15. Nemet AY, Vinker S. Considerations and complications after Bells’ palsy. J Clin Neurosci. 2015. 22: 1949-53
16. Oldenburg MS, Carlson ML, Van Abel KM, Driscoll CL, Link MJ. Management of geniculate ganglion hemangiomas: Case series and systematic review of the literature. Otol Neurotol. 2015. 36: 1735-40
17. Pulec JL. Facial nerve angioma. Ear Nose Throat J. 1996. 75: 225-38
18. Sataloff RT, Frattali MA, Myers DL. Intracranial facial neuromas: Total tumor removal with facial nerve preservation: A new surgical technique. Ear Nose Throat J. 1995. 74: 248-56
19. Semaan MT, Slattery WH, Brackmann DE. Geniculate ganglion hemangiomas: Clinical results and long-term follow-up. Otol Neurotol. 2010. 31: 665-70
20. Spiers AS. Syndrome of “crocodile tears”. Pharmacological study of a bilateral case. Br J Ophthalmol. 1970. 54: 330-4
21. Valença MM, Valença LP, Lima MC. Idiopathic facial paralysis (Bell’s palsy): A study of 180 patients. Arq Neuropsiquiatr. 2001. 59: 733-9
22. Wick CC, Sakai M, Richardson TE, Isaacson B. Transcanal endoscopic ear surgery for excision of a facial nerve venous malformation with interposition nerve grafting: A case report. Otol Neurotol. 2017. 38: 895-9
23. Yue Y, Jin Y, Yang B, Yuan H, Li J, Wang Z. Retrospective case series of the imaging findings of facial nerve hemangioma. Eur Arch Otorhinolaryngol. 2015. 272: 2497-503