- Katsuki Clinic, 21-3, Koganedai, Higashi-ward, Niigata, Japan.
DOI:10.25259/SNI_504_2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Masahito Katsuki, Shigemi Katsuki. A case of cardiac tamponade during the treatment of simultaneous cardio-cerebral infarction associated with atrial fibrillation – Case report. 06-Dec-2019;10:241
How to cite this URL: Masahito Katsuki, Shigemi Katsuki. A case of cardiac tamponade during the treatment of simultaneous cardio-cerebral infarction associated with atrial fibrillation – Case report. 06-Dec-2019;10:241. Available from: http://surgicalneurologyint.com/surgicalint-articles/9782/
Abstract
Background: Simultaneous cerebral and myocardial infarction is called cardio-cerebral infarction (CCI). It is a rare condition, and its management strategy has yet to be determined. We report a case of cardiac tamponade during the treatment of CCI associated with atrial fibrillation.
Case Description: A 72-year-old man presented with loss of consciousness after chest discomfort. He had taken rivaroxaban for paroxysmal atrial fibrillation. Twelve-lead electrocardiography showed ST elevation at II, III, and aVF. His National Institutes of Health Stroke Scale was 29. We diagnosed him with synchronous cardioembolic stroke and acute myocardial infarction due to atrial fibrillation. The coronary angiography revealed distal occlusion in the posterior descending branch of the right coronary artery, and overall myocardial perfusion seemed sufficient. The diffusion-weighted image showed hyperintense lesions at the cerebellum, and magnetic resonance angiography did not reveal the flow of the basilar artery. The patient’s NIH score improved immediately, so we did not perform intravenous tissue plasminogen activator (IV-tPA) administration nor endovascular treatment. Heparin administration was started. After 38 h from the onset, he suffered from hydrocephalus, and cerebral ventricular drainage was performed. Subsequently, circulatory dynamics worsened, and he was diagnosed with cardiac tamponade. Emergency pericardiotomy was performed, and he has been taking intensive care.
Conclusion: Some cases with CCI treated with IV-tPA and endovascular intervention were reported, but the treatment strategy should be still discussed multidisciplinary. Especially, the administration of antithrombotic drugs for CCI should be carefully performed because fatal hemorrhage such as cardiac tamponade can occur.
Keywords: Acute ischemic stroke, Acute myocardial infarction, Atrial fibrillation, Cardiac tamponade, Cardio- cerebral stroke
INTRODUCTION
Simultaneous cerebral and acute myocardial infarction (AMI) is called cardio-cerebral infarction (CCI)[
CASE PRESENTATION
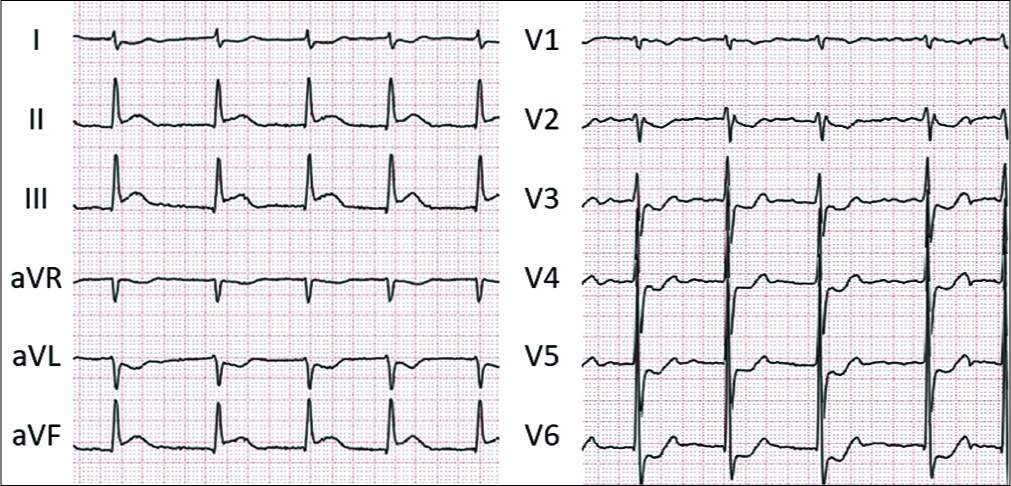
A 72-year-old man presented with sudden onset of loss of consciousness after chest discomfort and numbness in his left upper limb. His medical history included hypertension and paroxysmal atrial fibrillation, and he had taken rivaroxaban. His blood pressure was 150/101 mmHg without any significant difference between right and left limbs. The Japan coma scale was III-100, and the Glasgow Coma Scale was 8 (E1V2M5) on the admission. His National Institutes of Health Stroke Scale score was 29. Twelve-lead electrocardiography showed atrial fibrillation with a heart rate of 78 beats/min and ST elevation at II, III, and aVF and ST depression at V2– V6 [
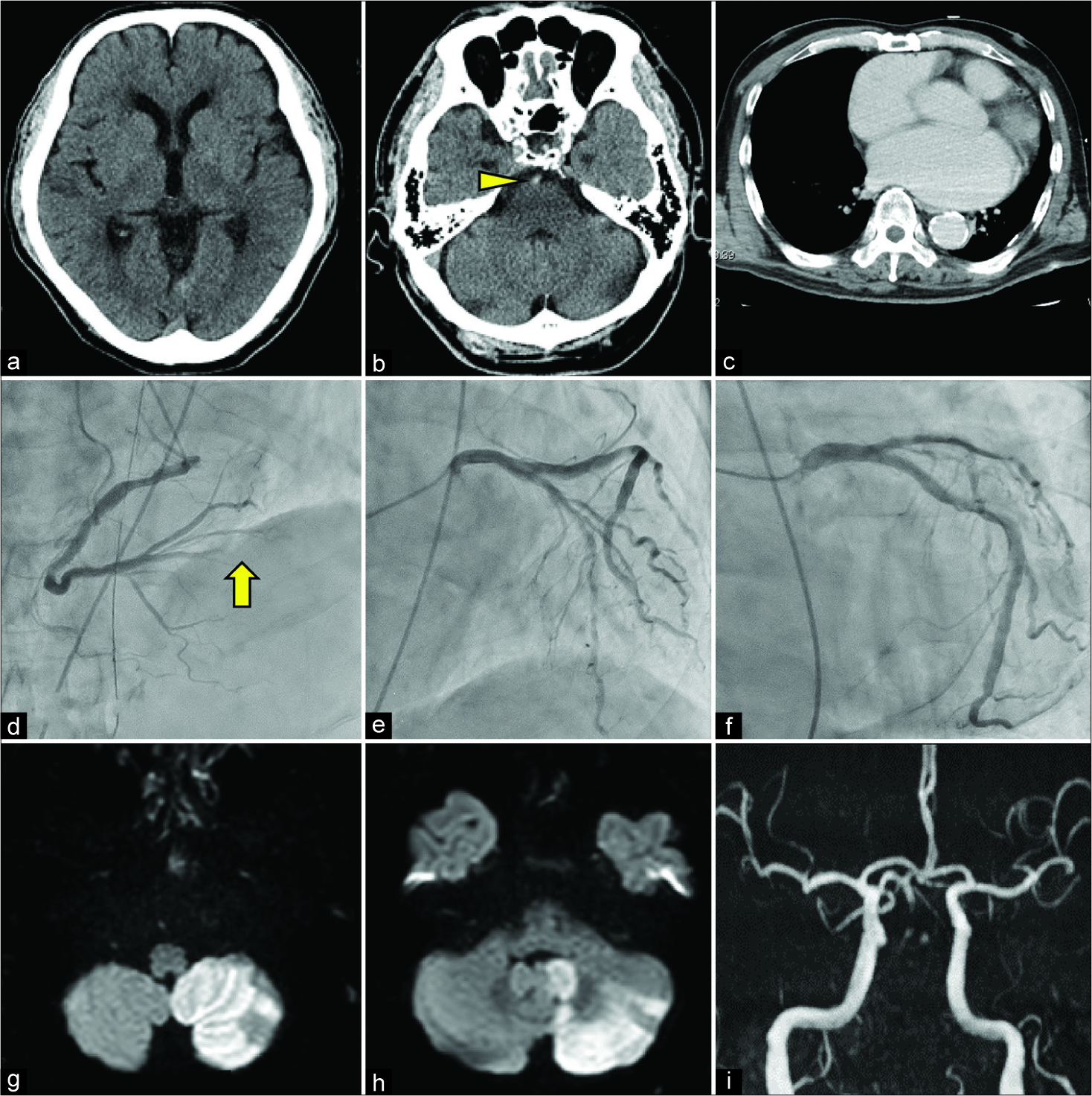
Figure 2:
The head computed tomography (CT) image showed no intracranial hemorrhages (a), but the hyper-dense arterial sign was observed at the basilar artery (b, arrowhead), which suggested the basilar artery occlusion. The contrast-enhanced chest CT did not indicate aortic dissection nor cardiac tamponade (c). The coronary angiography revealed distal occlusion in the posterior descending branch of the right coronary artery (d, arrow). Mild stenotic lesions suggestive of atherosclerotic pathology were observed in some parts (e and f). The diffusion-weighted image showed hyper-intense lesions in the region of the posterior inferior cerebellar artery (g and h). Magnetic resonance angiography did not reveal the flow of the basilar artery, and severe stenotic lesions of the major arteries were scarcely observed (i).
The patient was diagnosed with CCI, and a multidisciplinary discussion was done. Due to the current use of rivaroxaban and high NIH score, he was not considered as a candidate for intravenous tissue plasminogen activator (IV-tPA) treatment. We immediately performed coronary angiography for salvage of the myocardial ischemic area, and it revealed distal occlusion in the posterior descending branch of the right coronary artery [
After 38 h from the onset, his consciousness level was depressed as Japan coma scale I-3. The head CT showed hydrocephalus [
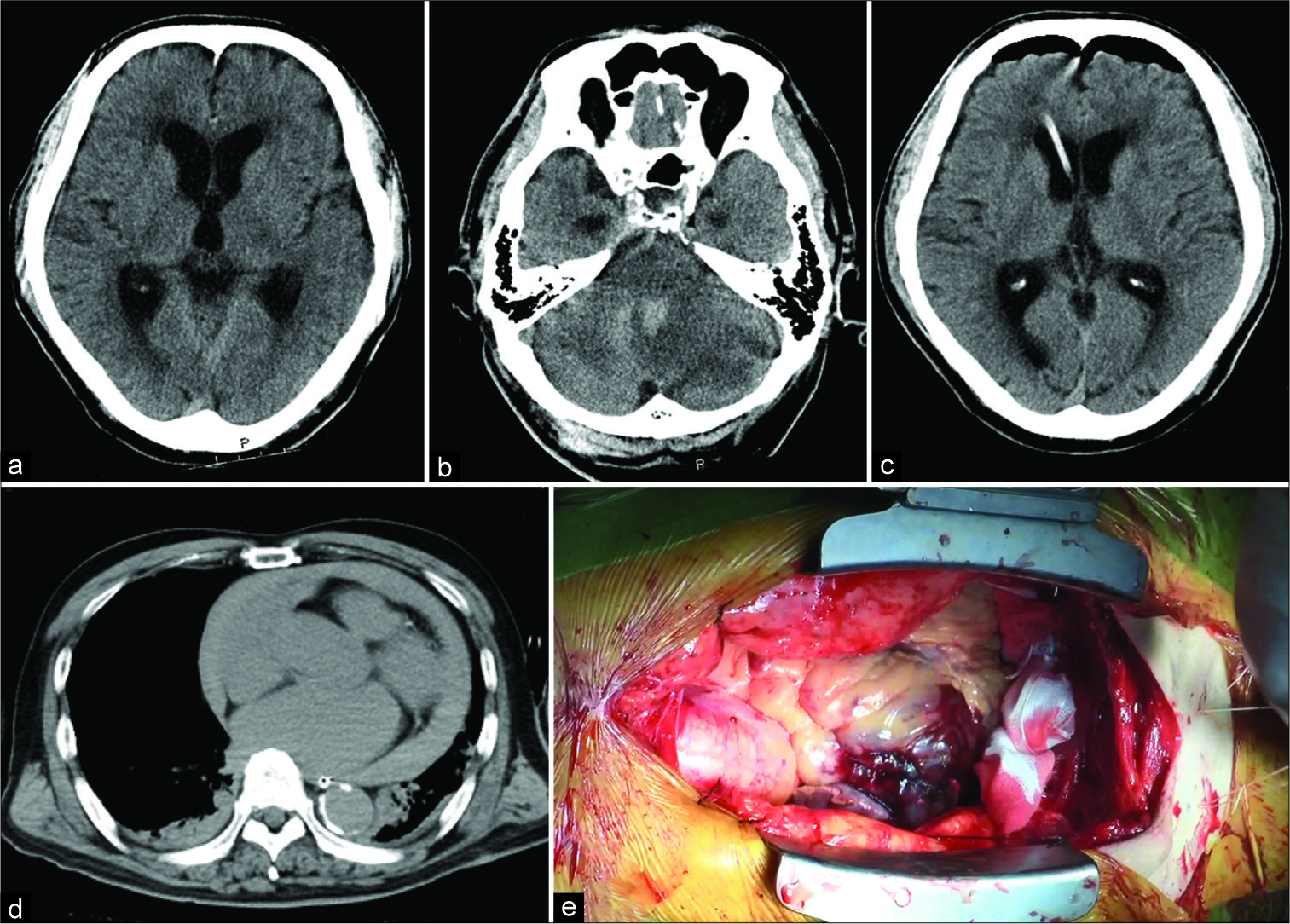
Figure 3:
After 38 h from the onset, his consciousness level was depressed as Japan coma scale I-3. The head computed tomography (CT) 38 h after the onset showed hydrocephalus (a) due to cerebellar edema and hemorrhagic infarction of the ischemic cerebellum, which compressed the aqueduct (b). The head CT after cerebral ventricular drainage showed improvement in hydrocephalus (c). The chest CT showed cardiac tamponade (d). Emergency pericardiotomy was immediately performed to allow the fluid to drain. A left mural and atrial appendage thrombi were observed. There were no apparent bleeding sources, aortic dissections, nor myocardial ruptures intraoperatively (e).
DISCUSSION
There are several mechanisms to explain CCI occurrence. The anterior and apical wall infarction associated with a decrease of the left ventricular systolic function induces the formation of the left ventricular mural thrombus,[
The American Heart Association and American Stroke Association in 2018 recommended that in the setting of hyper-acute simultaneous CCI, treatment with IV-tPA at the dose used for cerebral ischemia followed by PCI is reasonable (Class IIa; level of evidence C).[
Cases of cardiac tamponade during the treatment of acute cerebral infarction were reported, and the causes were aortic dissections and carcinomatous pericarditis.[
CONCLUSION
CCI is an infrequent and devastating clinical condition. Some cases with CCI treated with IV-tPA and endovascular intervention were reported, but the treatment strategy should be still discussed multidisciplinary. Especially, the administration of anticoagulant or antiplatelet for CCI should be carefully performed because fatal hemorrhage such as cardiac tamponade can occur.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
MK wrote this manuscript. SK approved the final manuscript. We are grateful to the patients and their families who willingly provided their medical data.
References
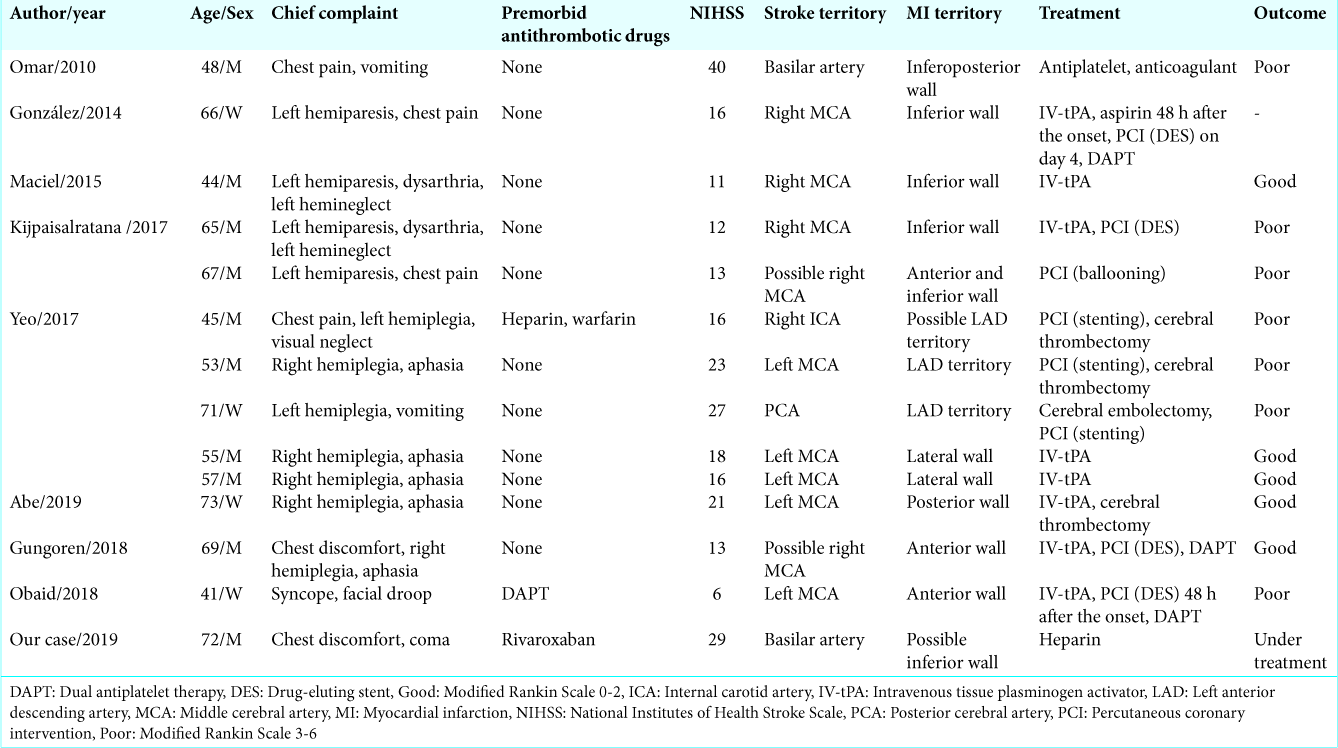
1. Abe S, Tanaka K, Yamagami H, Sonoda K, Hayashi H, Yoneda S. Simultaneous cardio-cerebral embolization associated with atrial fibrillation: A case report. BMC Neurol. 2019. 19: 152-
2. Bersano A, Melchiorre P, Moschwitis G, Tavarini F, Cereda C, Micieli G. Tako-tsubo syndrome as a consequence and cause of stroke. Funct Neurol. 2014. 29: 135-7
3. Chin PL, Kaminski J, Rout M. Myocardial infarction coincident with cerebrovascular accidents in the elderly. Age Ageing. 1977. 6: 29-37
4. Chlapoutakis GN, Kafkas NV, Katsanos SM, Kiriakou LG, Floros GV, Mpampalis DK. Acute myocardial infarction and transient ischemic attack in a patient with lone atrial fibrillation and normal coronary arteries. Int J Cardiol. 2010. 139: e1-4
5. Gianstefani S, Douiri A, Delithanasis I, Rogers T, Sen A, Kalra S. Incidence and predictors of early left ventricular thrombus after ST-elevation myocardial infarction in the contemporary era of primary percutaneous coronary intervention. Am J Cardiol. 2014. 113: 1111-6
6. González-Pacheco H, Méndez-Domínguez A, Vieyra-Herrera G, Azar-Manzur F, Meave-González A, Rodríguez-Zanella H. Reperfusion strategy for simultaneous ST-segment elevation myocardial infarction and acute ischemic stroke within a time window. Am J Emerg Med. 2014. 32: 1157.e1-4
7. Gungoren F, Besli F, Tanriverdi Z, Kocaturk O. Optimal treatment modality for coexisting acute myocardial infarction and ischemic stroke. Am J Emerg Med. 2019. 37: 795.e1-4
8. Kijpaisalratana N, Chutinet A, Suwanwela NC. Hyperacute simultaneous cardiocerebral infarction: Rescuing the brain or the heart first?. Front Neurol. 2017. 8: 664-
9. Maciel R, Palma R, Sousa P, Ferreira F, Nzwalo H. Acute stroke with concomitant acute myocardial infarction: Will you thrombolyse?. J Stroke. 2015. 17: 84-6
10. Nagasawa H, Ono H, Tanaka H, Yamakawa T. Case of cardiac tamponade during the treatment of acute cerebral infarction. Rinsho Shinkeigaku. 2014. 54: 218-22
11. Obaid O, Smith HR, Brancheau D. Simultaneous acute anterior ST-elevation myocardial infarction and acute ischemic stroke of left middle cerebral artery: A case report. Am J Case Rep. 2019. 20: 776-9
12. Omar HR, Fathy A, Rashad R, Helal E. Concomitant acute right ventricular infarction and ischemic cerebrovascular stroke; possible explanations. Int Arch Med. 2010. 3: 25-
13. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011. 365: 883-91
14. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019. 49: e46-110
15. Yeo LL, Andersson T, Yee KW, Tan BY, Paliwal P, Gopinathan A. Synchronous cardiocerebral infarction in the era of endovascular therapy: Which to treat first?. J Thromb Thrombolysis. 2017. 44: 104-11