- Division of Neurosurgery, Department of Surgery, Thammasat University Hospital, Faculty of Medicine, Thammasat University, Pathumthani, Thailand
Correspondence Address:
Raywat Noiphithak
Division of Neurosurgery, Department of Surgery, Thammasat University Hospital, Faculty of Medicine, Thammasat University, Pathumthani, Thailand
DOI:10.4103/2152-7806.195236
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Raywat Noiphithak, Gahn Doungprasert. A case of disseminated central nervous system sparganosis. 05-Dec-2016;7:
How to cite this URL: Raywat Noiphithak, Gahn Doungprasert. A case of disseminated central nervous system sparganosis. 05-Dec-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/a-case-of-disseminated-central-nervous-system-sparganosis/
Abstract
Background:Sparganosis is a very rare parasitic infection in various organs caused by the larvae of tapeworms called spargana. The larva usually lodges in the central nervous system (CNS) and the orbit. However, lumbar spinal canal involvement, as noted in the present case, is extremely rare. We report a rare case of disseminated CNS sparganosis involving the brain and spinal canal and review the literature.
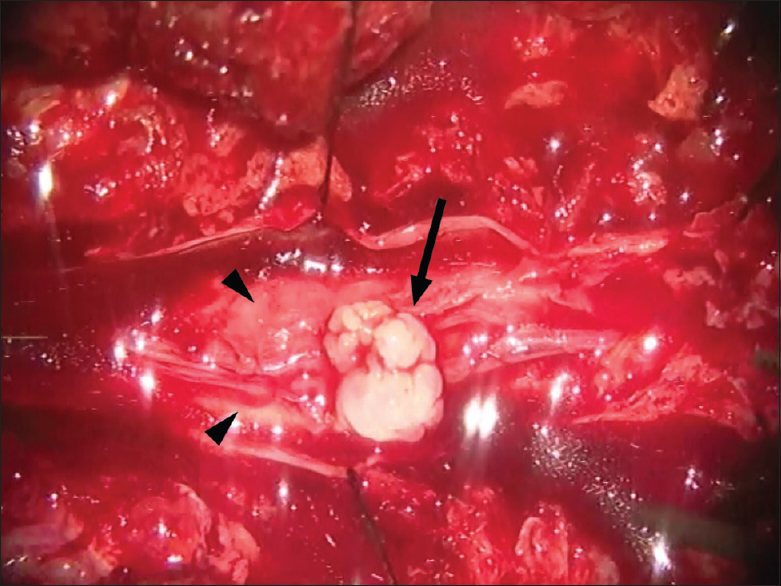
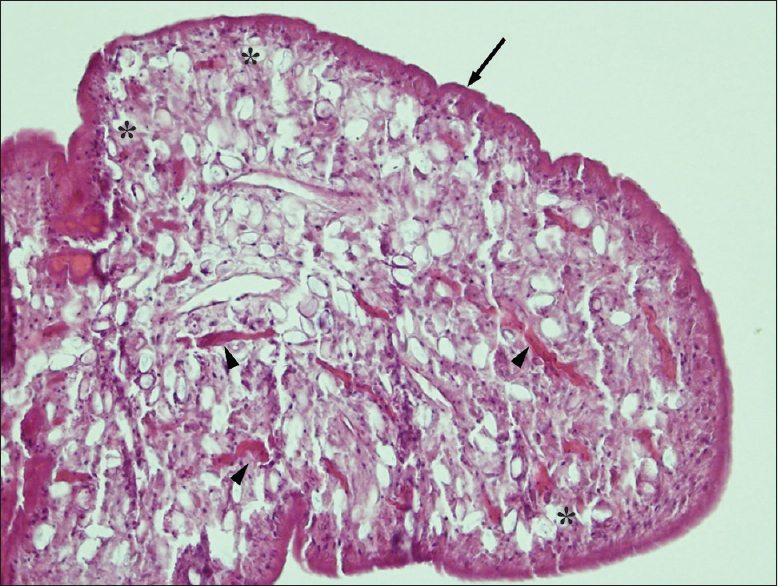
Case Description:A 54-year-old man presented with progressive low back pain and neurological deficit at the lumbosacral level for 2 months. Imaging indicated arachnoiditis and an abnormal lesion at the L4-5 vertebral level. The patient underwent laminectomy of the L4-5 with lesionectomy and lysis of adhesions between the nerve roots. Microscopic examination indicated sparganum infection. Further brain imaging revealed evidence of chronic inflammation in the left parieto-occipital area without evidence of live parasites. In addition, an ophthalmologist reported a nonactive lesion in the right conjunctiva. The patient recovered well after surgery, although he had residual back pain and bladder dysfunction probably due to severe adhesion of the lumbosacral nerve roots.
Conclusion:CNS sparganosis can cause various neurological symptoms similar to those of other CNS infections. A preoperative enzyme-linked immunosorbent assay is helpful for diagnosis, especially in endemic areas. Surgical removal of the worm remains the treatment of choice.
Keywords: CNS, diagnosis, disseminated, sparganosis, treatment
INTRODUCTION
Sparganosis is a parasitic infection caused by the pleocercoid larvae of pseudophyllidean tapeworms, which are called spargana.[
CASE REPORT
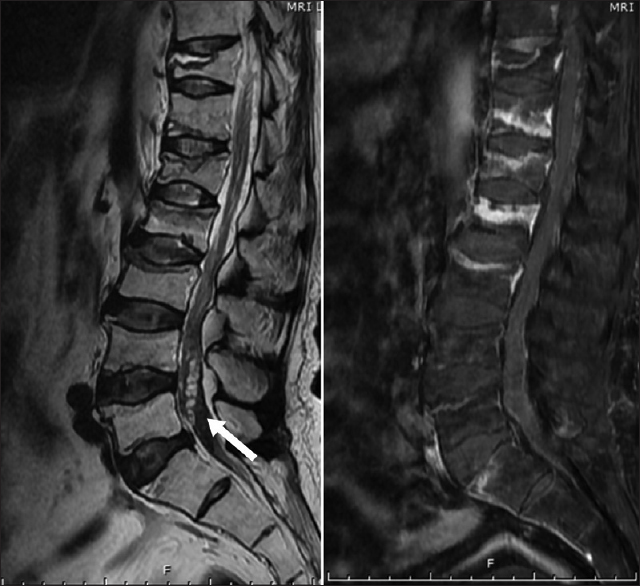
A 54-year-old Thai man presented with low back pain lasting for 2 months. The symptom was gradually progressive and was aggravated by movements. The pain was referred to both legs and did not improve with medications. Later, the patient experienced weakness and numbness in his right leg and had walking difficulties. In addition, he had urinary retention which required urinary catheterization. The patient's symptoms worsened and limited his daily activities. Examination revealed generalized erythematous plaques on his head, trunk, back, and extremities. He also had several flaccid blisters. The patient had grade III motor weakness and paresthesia in his entire right leg; however, there was minimal motor weakness on the left side. Sacral sensation and sphincter tone were intact. Magnetic resonance imaging (MRI) of the lumbar spine showed a pattern of arachnoiditis at the L1-5 level. There were also multiple hypersignal intensity bead-like lesions on T2-weighted images in the spinal canal of the L4-5 vertebral level. The lesions were not enhanced with contrast [
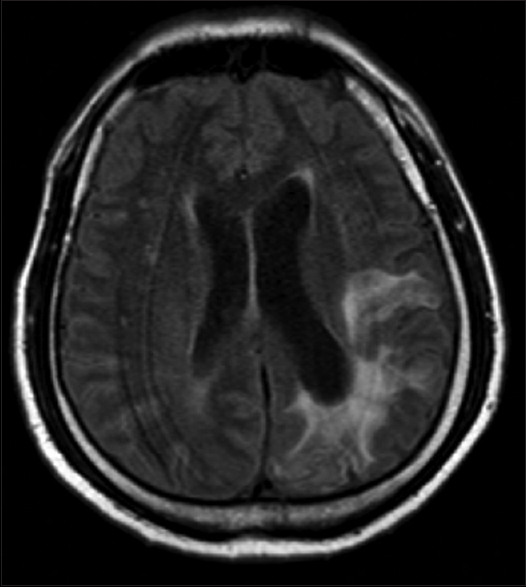
The patient was a monk on pilgrimage and had ingested uncooked frogs, snakes, and other amphibians for 10 years. The year prior to his visit he was diagnosed with bullous pemphigoid and was on high-dose prednisolone since then. Further investigation was done to rule out potentially disseminated sparganosis. In addition, the patient had a history of epilepsy, which was neither investigated nor treated. Although brain MRI indicated cerebral atrophy with surrounding hypersignal intensity in the left parieto-occipital area on T2-weighted images, which is compatible with old inflammation, there was no evidence of the live parasite in the brain [
In the early postoperative period, the patient still had severe back pain; however, this improved during follow-up after a few months. Radicular pain, and weakness were also improved; however, neurological function of the urinary bladder was still deficit.
DISCUSSION
Sparganosis is a rare parasitic infection caused by larvae of the parasitic tapeworms of Spirometra.[
Human sparganosis can occur in various organs. The most common sites of infection, as indicated in a publication from Thailand, are the eye, subcutaneous tissue, and the CNS.[
Computed tomography and MRI may be helpful; however, these are nonspecific tools for detection of CNS sparganosis.[
Antiparasitic drugs for tapeworm, such as praziquantel, are ineffective for the treatment CNS sparganosis.[
CONCLUSION
Although sparganosis is a very rare CNS infection, it can cause various neurological symptoms and should be considered as a differential diagnosis. In addition to history taking and imaging, preoperative ELISA can be helpful for the diagnosis of sparganosis, especially in endemic areas. Surgical removal of the worm is the treatment of choice and has good outcomes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We are grateful to Waiwit Sanguanwongwarn, M.D. at Department of Orthopedic Surgery, Huahin Hospital, Prachuap Khiri Khan, Thailand, for support in clinical information of the patient, and Dussadee Sakonlaya, M.D. at Department of Pathology, Thammasat University Hospital, Pathumthani, Thailand, for support in the pathological analyses.
References
1. Andersen KI. Description of musculature differences in spargana of Spirometra (Cestoda; Pseudophyllidea) and tetrathyridia of Mesocestoides (Cestoda; Cyclophyllidea) and their value in identification. J Helminthol. 1983. 57: 331-4
2. Bao XY, Ding XH, Lu YC. Sparganosis presenting as radiculalgia at the conus medullaris. Clin Neurol Neurosurg. 2008. 110: 843-6
3. Beaver PC.editorsClinical Parasitology. Philadelphia: Lea & Febiger; 1984. p.
4. Chang KH, Han MH. MRI of CNS parasitic diseases. J Magn Reson Imaging. 1998. 8: 297-307
5. Cho SY, Kang SY, Kong Y. Purification of antigenic protein of sparganum by immunoaffinity chromatography using a monoclonal antibody. Korean J Parasitol. 1990. 28: 135-
6. Cobbold T. Description of Ligula mansoni, a new human cestode. Zoo J Linn Soc Lond. 1883. 17: 78-
7. Kim H, Kim SI, Cho SY. Serological Diagnosis Of Human Sparganosis By Means Of Micro-ELISA. Kisaengchunghak Chapchi. 1984. 22: 222-8
8. Kudesia S, Indira DB, Sarala D, Vani S, Yasha TC, Jayakumar PN. Sparganosis of brain and spinal cord: Unusual tapeworm infestation (report of two cases). Clin Neurol Neurosurg. 1998. 100: 148-52
9. Kwon J, Kim JS. Sparganosis presenting as a conus medullaris lesion: Case report and literature review of the spinal sparganosis. Arch Neurol. 2004. 61: 1126-8
10. Mueller JF, Hart EP, Walsh WP. Human Sparganosis in the United States. J Parasitol. 1963. 49: 294-6
11. Park HY, Lee SU, Kim SH, Lee PC, Huh S, Yang YS. Epidemiological significance of sero-positive inhabitants against sparganum in Kangwon-do, Korea. Yonsei Med J. 2001. 42: 371-4
12. Park JH, Park YS, Kim JS, Roh SW. Sparganosis in the Lumbar Spine: Report of Two Cases and Review of the Literature. J Korean Neurosurg Soc. 2011. 49: 241-4
13. Roh SY, Lee JY, Park KW, Jung S-N. Sparganosis in a patient with diffuse large B cell lymphoma. J Cancer Res Ther. 2013. 9: 712-4
14. Song T, Wang WS, Zhou BR, Mai WW, Li ZZ, Guo HC. CT and MR Characteristics of Cerebral Sparganosis. Am J Neuroradiol. 2007. 28: 1700-5
15. Sundaram C, Prasad V, Reddy JJ. Cerebral sparganosis. J Assoc Physicians India. 2003. 51: 1107-10
16. Tang TW, Huang JS, Huang SH, Su KE, Chang YL. Sparganosis of the Spinal Canal: Rare Tapeworm Infection as a Cauda Equina Mass with Magnetic Resonance Imaging. J Radiol Sci. 2001. 36: 139-44
17. Wiwanitkit V. A review of human sparganosis in Thailand. Int J Infect Dis. 2005. 9: 312-6