- Department of Neurosurgery, Kyoto Prefectural University of Medicine, Japanese Red Cross Society, Kyoto Daini Hospital, Kyoto, Japan.
- Department of Diagnostic Pathology, Nishinotoin Bukkoji Clinic, Japanese Red Cross Society, Kyoto Daini Hospital, Kyoto, Japan.
- Department of Neurosurgery, Japanese Red Cross Society, Kyoto Daini Hospital, Kyoto, Japan.
- Department of Pathology, Japanese Red Cross Society, Kyoto Daini Hospital, Kyoto, Japan.
Correspondence Address:
Gaku Fujiwara, Department of Neurosurgery, Kyoto Prefectural University of Medicine, Kyoto, Japan.
DOI:10.25259/SNI_1193_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Gaku Fujiwara1, Daisuke Maruyama1, Hidetosho Okabe2, Yujiro Komaru3, Mamoru Murakami3, Kanade Katsura4, Nobukuni Murakami3, Naoya Hashimoto1. A case of fibromuscular dysplasia related intracerebral hemorrhage without angiographically cerebral abnormal vessels. 20-Jan-2022;13:26
How to cite this URL: Gaku Fujiwara1, Daisuke Maruyama1, Hidetosho Okabe2, Yujiro Komaru3, Mamoru Murakami3, Kanade Katsura4, Nobukuni Murakami3, Naoya Hashimoto1. A case of fibromuscular dysplasia related intracerebral hemorrhage without angiographically cerebral abnormal vessels. 20-Jan-2022;13:26. Available from: https://surgicalneurologyint.com/surgicalint-articles/11350/
Abstract
Background: Fibromuscular dysplasia (FMD) can cause cerebral aneurysms and dissection, which can lead to stroke. Angiographic findings are important in the diagnosis. We report a case of FMD in which the cause of hemorrhage could not be determined by angiography.
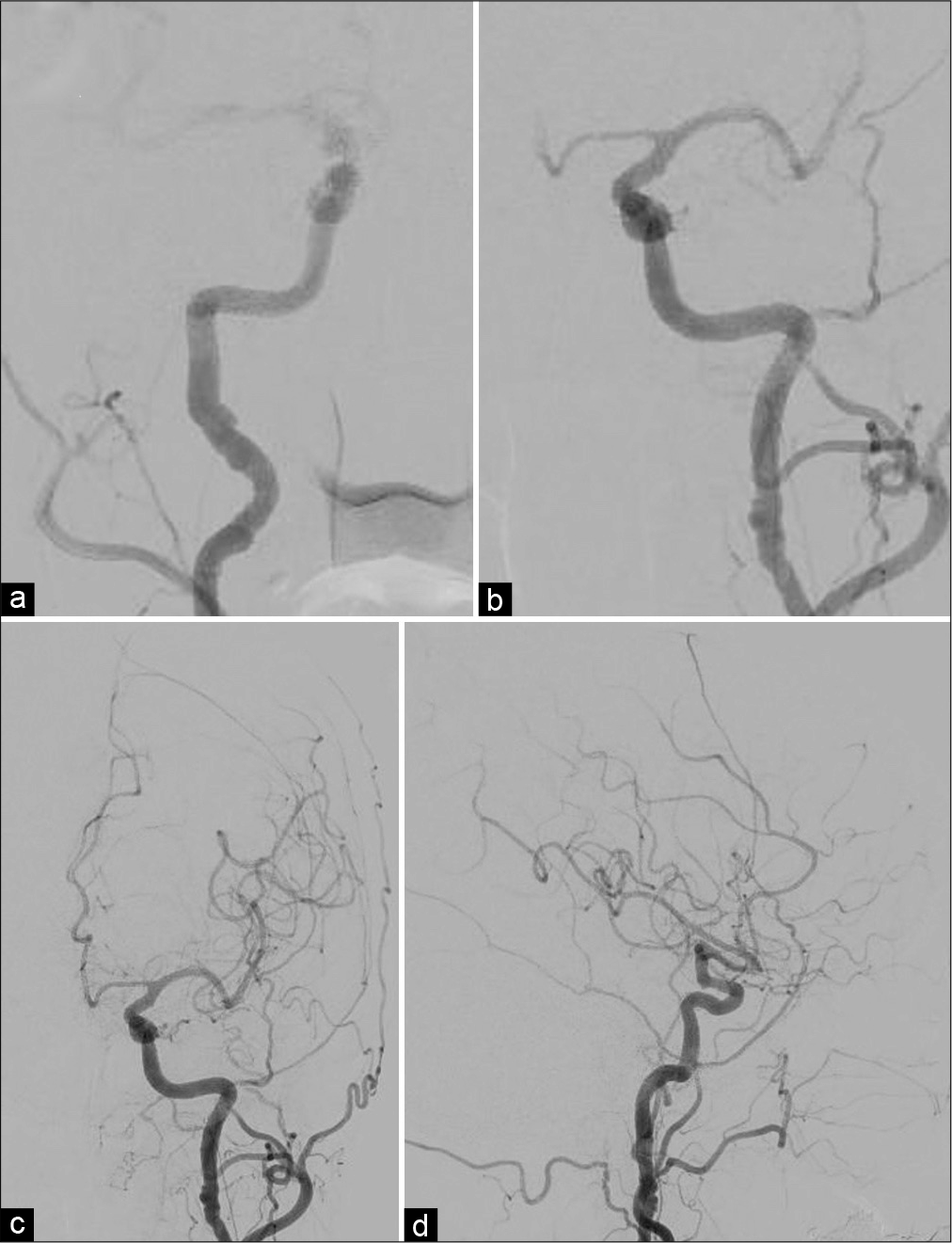
Case Description: A 73-year-old woman suffered from intracerebral hemorrhage (ICH) associated with FMD without abnormal angiography cerebral vessels. She presented with headache and nausea. Subsequent head-computed tomography-revealed ICH in the left frontal lobe, and contrast-enhanced magnetic resonance imaging revealed a gadolinium-enhancing lesion in the perihematoma area and in the genu of the corpus callosum. Although cerebral angiography revealed a string of beads appearance in the bilateral extracranial internal carotid arteries, no abnormality explaining the hemorrhage was identified. The hematoma was removed and the pathological diagnosis was FMD. In the pathological specimen, various patterns of vulnerable vessels, such as aneurysmal dilatation and obstruction, were observed, which could easily collapse and result in hemorrhage. In the case of ICH of unknown origin, microscopic vessel disruption due to FMD should also be considered.
Conclusion: FMD can cause ICH in microscopic vascular lesions that are undetectable on angiography.
Keywords: Fibromuscular dysplasia, Intracerebral hemorrhage, Stroke
INTRODUCTION
Fibromuscular dysplasia (FMD) is a noninflammatory and nonatherosclerotic vascular disorder that leads to arterial tortuosity, stenosis, occlusion, aneurysm, and dissection of the middle and distal arterial segments of the human body.[
CASE REPORT
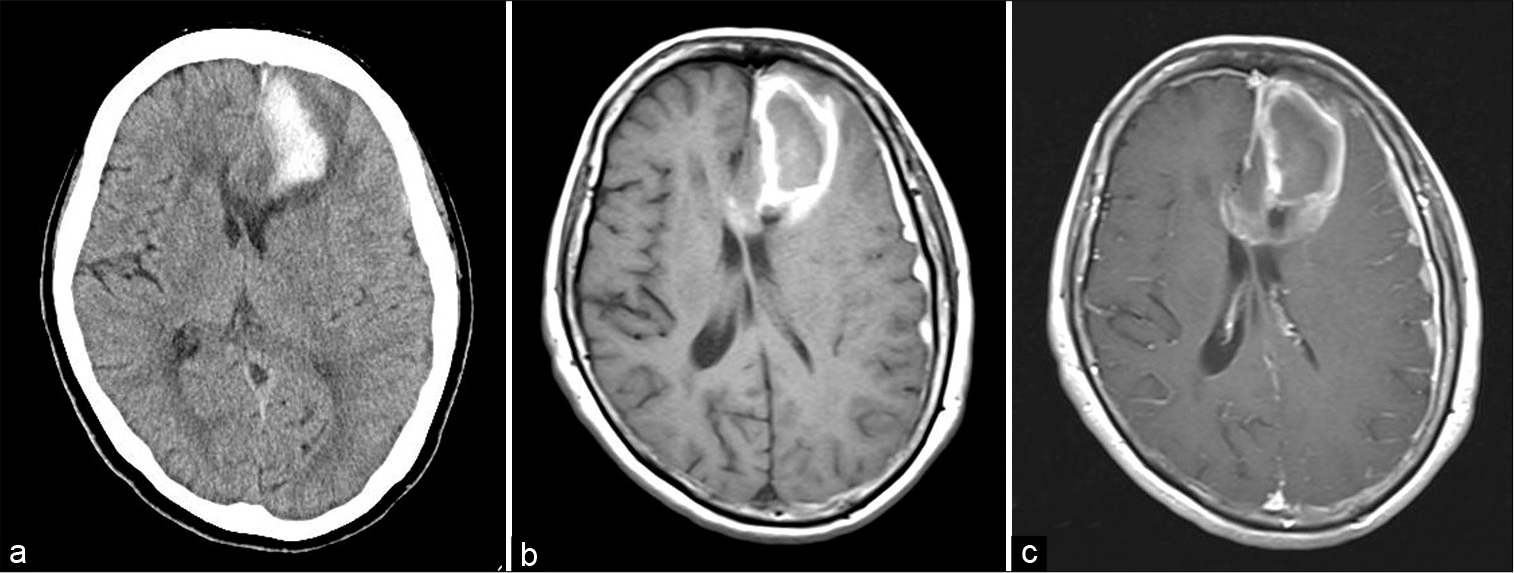
A 73-year-old Japanese woman with a history of hyperlipidemia, diabetes mellitus, and malignant lymphoma of mucosa-associated lymphoid tissue (MALT) presented to our hospital complaining of headache and nausea for 10 days. MALT lymphoma had been treated with chemotherapy 10 years ago and was in complete remission. The patient had no antithrombotic medications. The patient was fully alert and had no neurological deficits on initial physical examination. Brain computed tomography (CT) and magnetic resonance imaging (MRI) demonstrated subacute subcortical hematoma mainly in the left superior to middle frontal gyrus (43 × 18 × 44 mm) with gadolinium enhancement in the perihematoma area and in the genu of the corpus callosum [
Figure 3:
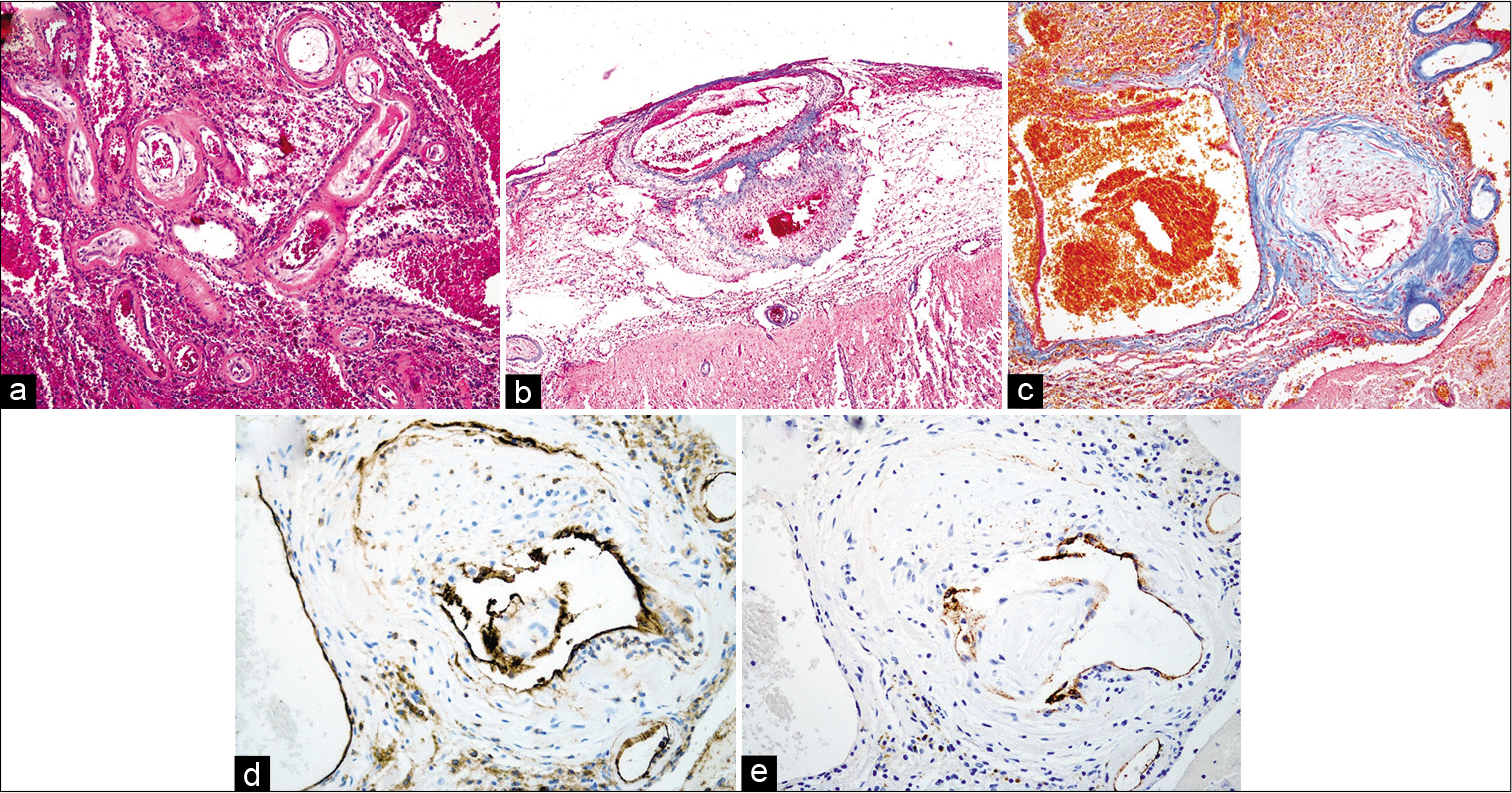
Continuous stenosis with multifocal obliterations in the segment of small arterial fibromuscular dysplasia (FMD) affecting media to intima (a), and recanalization was shown in some of these obliterations. Consecutive two aneurysms occurring within the arterial wall with characteristic medial FMD (b) and markedly expanded aneurysm with quite thin residual wall locating nearby the artery with stenosis due to FMD (c). Areas of wall thickening and greatly dilated vessel endothelium are CD31-positive (d), whereas the endothelium of the thin-walled aneurysm is CD34-negative (e). (Original magnifications ×40 (a-c), ×100 (d and e)).
DISCUSSION
ICH due to FMD without obvious angiography abnormal vessels is an extremely rare entity and characteristic pathological findings are presented in this case. First, ICH associated with FMD has been reported in some reports [
According to the previous pathological reports of FMD, it was reported that FMD has lesions in small- and medium- sized vessels that cannot be captured on imaging.[
CONCLUSION
We encountered a rare case of FMD that caused ICH without angiography cerebral abnormal vessels and showed marked pathological findings in the small vessel wall. It should be considered that FMD can cause ICH due to microscopic obstruction or aneurysmal dilatation caused by vessel wall vulnerability.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bellot J, Gherardi R, Poirier J, Lacour P, Debrun G, Barbizet J. Fibromuscular dysplasia of cervico-cephalic arteries with multiple dissections and a carotid-cavernous fistula. A pathological study. Stroke. 1985. 16: 255-61
2. Gornik HL, Persu A, Adlam D, Aparicio LS, Azizi M, Boulanger M. First international consensus on the diagnosis and management of fibromuscular dysplasia. Vasc Med (United Kingdom). 2019. 24: 164-89
3. Imakita S, Nishimura T, Yamada N, Naito H, Takamiya M, Yamada Y. Magnetic resonance imaging of cerebral infarction: Time course of Gd-DTPA enhancement and CT comparison. Neuroradiology. 1988. 30: 372-8
4. Kadian-Dodov D, Gornik HL, Gu X, Froehlich J, Bacharach JM, Chi YW. Dissection and aneurysm in patients with fibromuscular dysplasia: Findings from the U. S registry for FMD. J Am Coll Cardiol. 2016. 68: 176-85
5. Krittanawong C, Kumar A, Johnson KW, Kaplin S, Virk HU, Wang Z. Prevalence, presentation, and associated conditions of patients with fibromuscular dysplasia. Am J Cardiol. 2019. 123: 1169-72
6. Mettinger KL, Ericson K. Fibromuscular dysplasia and the brain. I Observations on angiographic, clinical and genetic characteristics. Stroke. 1982. 13: 46-52
7. Olin JW, Sealove BA. Diagnosis, management, and future developments of fibromuscular dysplasia. J Vasc Surg. 2011. 53: 826-36.e1
8. Ooba H, Takeda Y, Kato Y, Maruiwa H, Kobayashi H. Intracranial atypical fibromuscular dysplasia with ruptured aneurysm--case report. Neurol Med Chir (Tokyo). 2004. 44: 540-3
9. Osborn AG, Anderson RE. Angiographic spectrum of cervical and intracranial fibromuscular dysplasia. Stroke. 1977. 8: 617-26
10. Pappaccogli M, Di Monaco S, Warchoł-Celińska E, Lorthioir A, Amar L, Aparicio LS. The European/ international fibromuscular dysplasia registry and initiative (FEIRI) clinical phenotypes and their predictors based on a cohort of 1000 patients. Cardiovasc Res. 2021. 117: 950-9
11. Pasquini M, Trystram D, Nokam G, Gobin-Metteil MP, Oppenheim C, Touzé E. Fibromuscular dysplasia of cervicocephalic arteries: Prevalence of multisite involvement and prognosis. Rev Neurol (Paris). 2015. 171: 616-23
12. Persu A, Giavarini A, Touzé E, Januszewicz A, Sapoval M, Azizi M. European consensus on the diagnosis and management of fibromuscular dysplasia. J Hypertens. 2014. 32: 1367-78
13. Pronin IN, Holodny AI, Petraikin A V. MRI of high-grade glial tumors: Correlation between the degree of contrast enhancement and the volume of surrounding edema. Neuroradiology. 1997. 39: 348-50
14. Pusztaszeri MP, Seelentag W, Bosman FT. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J Histochem Cytochem. 2006. 54: 385-95
15. Seevinck PR, Deddens LH, Dijkhuizen RM. Magnetic resonance imaging of brain angiogenesis after stroke. Angiogenesis. 2010. 13: 101-11
16. Shibata Y, Sugimoto K, Onizuka H, Matsuki T, Nose T. Gadolimium-DTPA-enhanced MR imaging of cerebral infections. Jpn J Neurosurg. 1993. 2: 23-8
17. Slovut DP, Olin JW. Fibromuscular dysplasia. N Engl J Med. 2004. 350: 1862-71
18. Zagzag D, Goldenberg M, Brem S. Angiogenesis and blood-brain barrier breakdown modulate CT contrast enhancement: An experimental study in a rabbit brain-tumor model. Am J Roentgenol. 1989. 153: 141-6
19. Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and dysfunction of the blood-brain barrier. Cell. 2015. 163: 1064-78