- Department of Neurosurgery, Albert Einstein College of Medicine, New York, United States.
- Department of Radiology, Montefiore Medical Center, Bronx, New York, United States.
- Department of Neurosurgery, Montefiore Medical Center, Bronx, New York, United States.
Correspondence Address:
Samuel Jack Ahmad, Albert Einstein College of Medicine, Bronx, New York, United States.
DOI:10.25259/SNI_313_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Samuel Jack Ahmad1, Richard L. Zampolin2, Allan L. Brook2, Andrew J. Kobets3, David J. Altschul3. A case of hydrocephalus confounded by suprasellar arachnoid cyst and concomitant reversible cerebral vasoconstriction syndrome. 29-Jul-2022;13:331
How to cite this URL: Samuel Jack Ahmad1, Richard L. Zampolin2, Allan L. Brook2, Andrew J. Kobets3, David J. Altschul3. A case of hydrocephalus confounded by suprasellar arachnoid cyst and concomitant reversible cerebral vasoconstriction syndrome. 29-Jul-2022;13:331. Available from: https://surgicalneurologyint.com/surgicalint-articles/11746/
Abstract
Background: Obstructive hydrocephalus is a neurologic condition that has varied clinical and imaging presentations, as well as a multitude of congenital etiologies including aqueductal stenosis and less commonly arachnoid cysts. Aqueductal stenosis is a physical limitation to cerebrospinal fluid flow along the course of the aqueduct, which results in enlargement of the third and lateral ventricles. Arachnoid cysts are thin walled and fluid filled central nervous system lesions that can result in mass effect on adjacent structures. While arachnoid cysts are mostly asymptomatic, they may present with neurological symptoms that vary depending on the location of the lesion. Suprasellar cysts in particular may cause obstructive hydrocephalus as well as endocrine dysfunction. Reversible cerebral vasoconstriction syndrome (RCVS) is an unusual condition caused by cerebral arterial vasoconstriction that often presents initially with a thunderclap headache. Frequently, there is some environmental trigger associated with this condition. RCVS more commonly affects women and can induce stroke.
Case Description: A 57-year-old female presented to the emergency department with progressive headache and visual changes. Initial workup suggested the patient’s symptoms where related to RCVS but subsequent surgical management of what was presumed to be long standing, compensated hydrocephalus resulted in resolution of the patient’s symptoms.
Conclusion: We report, to the best of our knowledge, the first case of aquedutal stenosis and suprasellar arachnoid cyst with concomitant RCVS. The presence of multiple pathologies found on radiologic imaging illustrates the challenges presented by incidental findings and subsequent anchoring bias in medical diagnosis.
Keywords: Aqueductal stenosis, Endoscopic third ventriculostomy, Reversible cerebral vasoconstriction syndrome, Suprasellar arachnoid cyst
INTRODUCTION
Obstructive hydrocephalus, also known as non-communicating hydrocephalus, is caused by the impedance of cerebrospinal fluid (CSF) flow within the ventricles. Patients with obstructive hydrocephalus may suffer from headache, nausea, vision abnormalities, and altered gait. Obstructive hydrocephalus most commonly results following infection and hemorrhage, but there exist a multitude of congenital etiologies including aqueductal stenosis and arachnoid cysts. Aqueductal stenosis most frequently affects the proximal portion of the aqueduct, but also can occur distally, with a web or membrane of tissue obstructing flow into the fourth ventricle.[
Primary arachnoid cysts are thin-walled congenital lesions derived from the arachnoid mater. These cysts are comprised of meningothelial cells that secrete fluid similar to CSF. However, these cysts do not in fact communicate with either the subarachnoid space or ventricles.[
During the work-up of patients with nonspecific neurologic presentations such as headache and visual changes, confusion as to the underlying causative etiology can be encountered when diagnostic testing yields findings that have overlapping symptomatology. Careful diagnostic decision-making with awareness of the pitfalls of anchoring bias is necessary to ultimately achieve the correct diagnosis and treatment. In the workup for headache, finding evidence of subarachnoid hemorrhage (SAH) results in well-established differential pathways that require the exclusion of specific dangerous etiologies such as aneurysm and vascular malformations. Once excluded from the study, less common etiologies such as reversible cerebral vasoconstriction syndrome (RCVS) can be considered.[
Herein, we present, to the best of our knowledge, the first reported case of aqueductal stenosis confounded by a suprasellar arachnoid cyst and concomitant RCVS.
CASE PRESENTATION
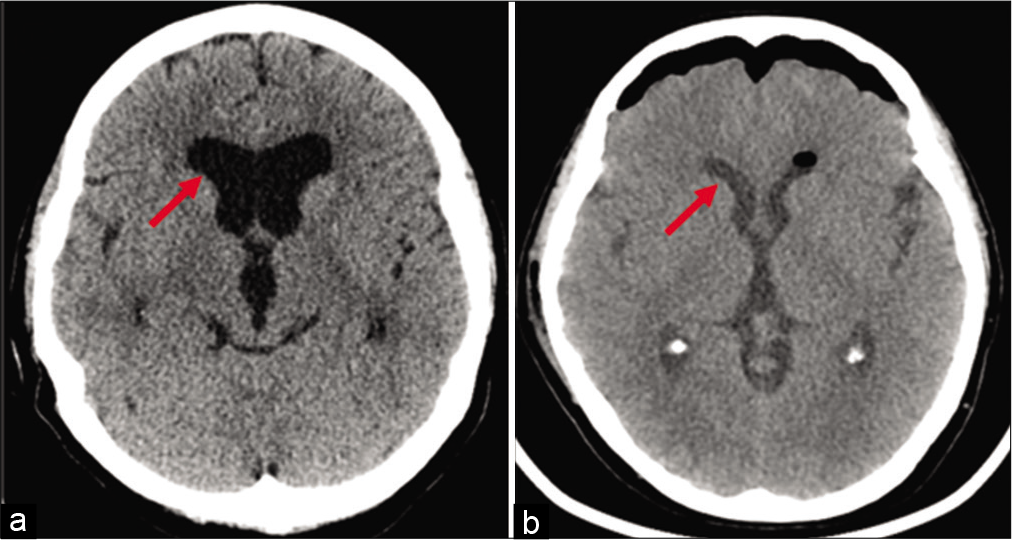
A 57-year-old female with a medical history of hypertension, hyperlipidemia, depression, and anxiety presented to the emergency department (ED) with progressive frontal headache 2–3 weeks in duration. Her headache was associated with transient blurry vision. The patient denied nausea, photophobia, neck pain, or altered gait. Head computed tomography (CT) showed enlargement of the lateral and third ventricles, empty sella turcica without transependymal resorption of CSF, or acute intracranial hemorrhage. Findings were consistent with chronic hydrocephalus [
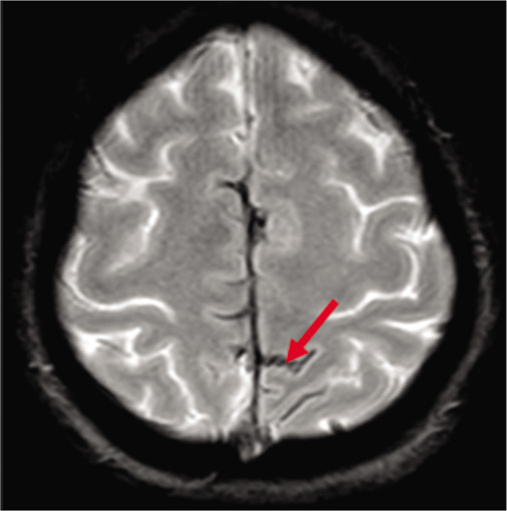
Magnetic resonance imaging (MRI) of the brain demonstrated chronic cortical SAH (cSAH)/superficial siderosis of the parasagittal frontoparietal sulci and left anterior frontal sulci [
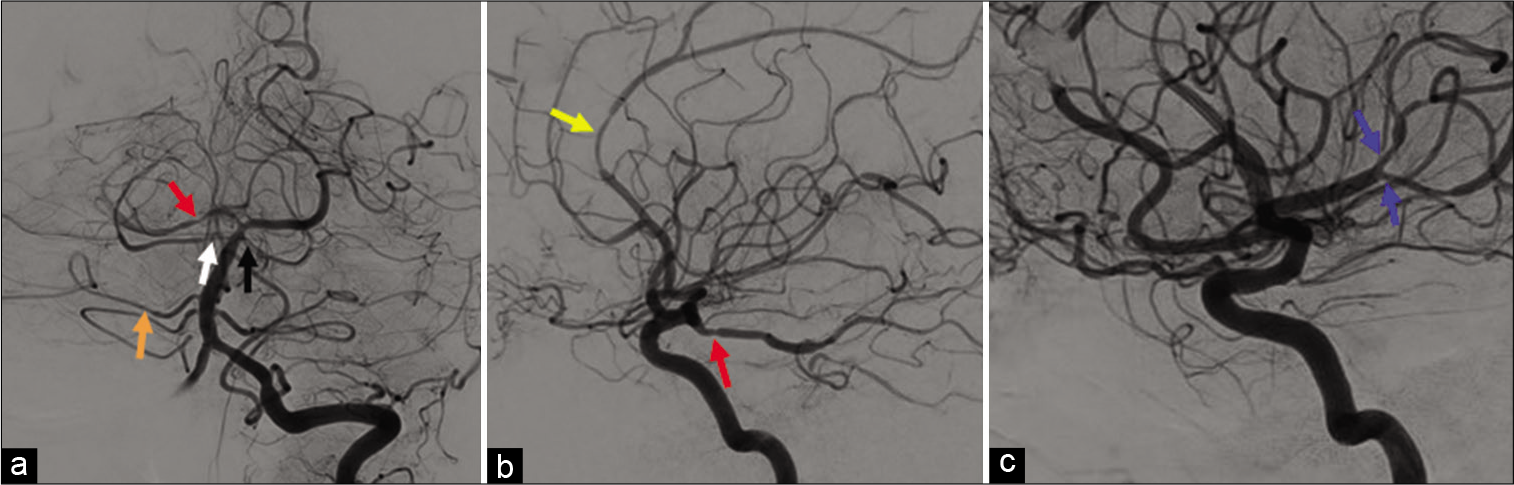
Figure 3:
Arrows point to multifocal stenosis of medium to small caliper cerebral arteries demonstrated on angiography. (a) Red arrow: R. posterior cerebral artery; White arrow: R. superior cerebellar artery; Orange arrow: R. anterior inferior cerebellar artery; Black arrow: L. superior cerebellar. (b) Yellow arrow: R. anterior cerebral artery; Red arrow: R. posterior cerebral artery. (c) Blue arrows: L. middle cerebral artery.
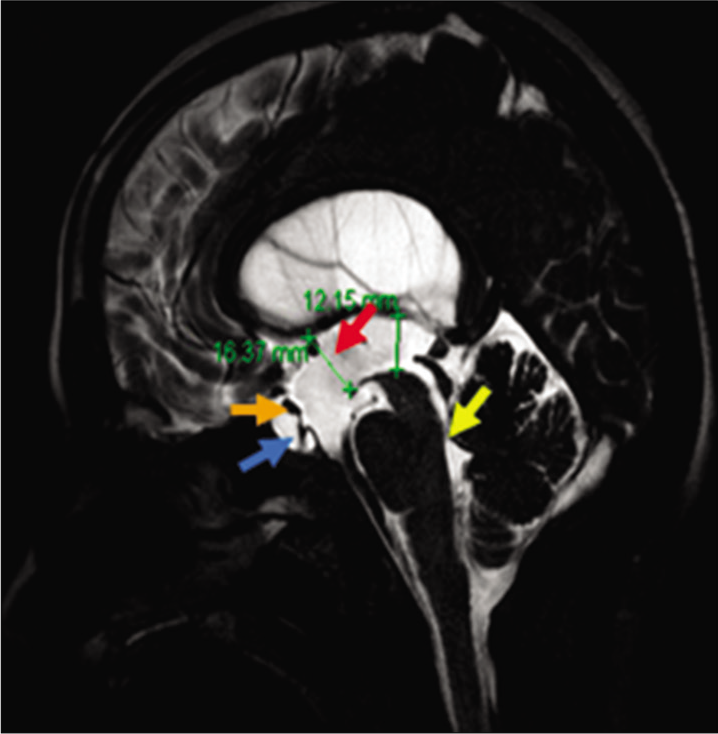
An MRI of the brain with CSF flow study was subsequently performed, revealing partial obstruction of the cerebral aqueduct [
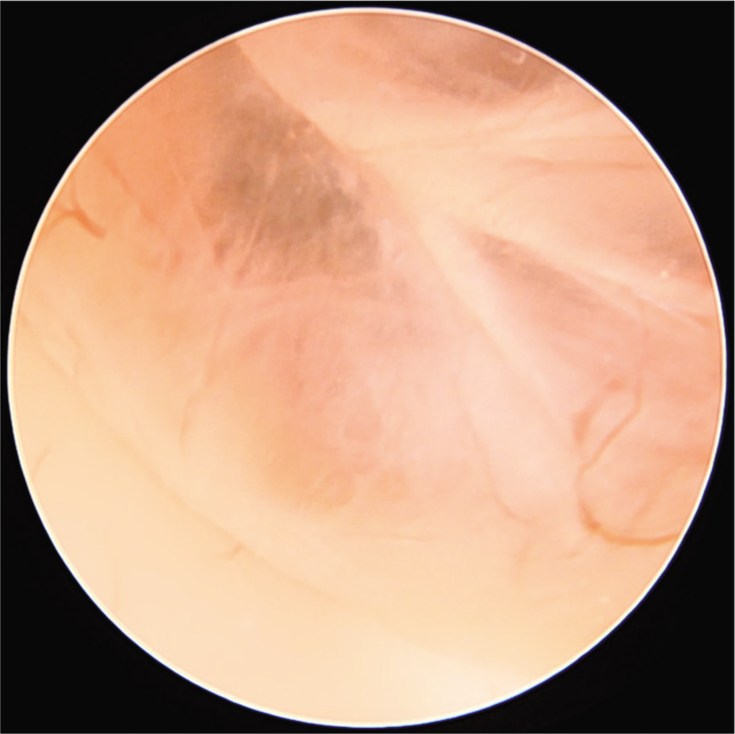
ETV with the right frontal brain biopsy was performed. Intraoperative endoscopy located a large cyst within the third ventricle obscuring the ventricular floor [
CSF studies were negative for malignancy and bacterial, viral, or fungal infection. Her CSF total protein level was found to be low, with a concentration of <10 mg per dL. Her CSF was negative for white blood cells (WBCs), but contained 600 red blood cells (RBCs) per µ˄. She was also found to be antinuclear antibody negative. Postoperative head CT demonstrated a significant decrease in hydrocephalus [
DISCUSSION
The subject of this case study was found to have three concomitant conditions, aqueductal stenosis, suprasellar arachnoid cyst, and RCVS following diagnostic workup and subsequent surgical management with ETV. The initial workup of this patient on ED presentation resulted in a brain MRI demonstrating cortical SAH and a CTA showing multifocal intracranial arterial stenoses. These findings were initially assumed to explain the patient’s clinical syndrome and a diagnosis of RCVS was presumed as other etiologies of cSAH and multifocal arterial stenosis were excluded from the study. The finding of compensated hydrocephalus as well as mass effect within the suprasellar cistern was given less weight in the diagnostic decision making, ultimately resulting in a delayed diagnosis and treatment. In this way, the findings of cSAH and vascular stenoses were used to anchor the diagnostic workup and thinking about this patient’s condition until other evidence dislodged these initial assumptions of causality.
RCVS is thought to originate from abnormally elevated cerebral arterial tone due to excessive sympathetic output and neuronal stimulus.[
Reperfusion injury of the leptomeningeal arteries may result in cSAHs, which have been found in 22% of RCVS cases.[
RCVS must be differentiated from primary angiitis of the central nervous system (PACNS), an idiopathic vasculitis limited to the medium-sized and small arteries of the central and peripheral nervous systems. PACNS causes headache, paresis, and other nonspecific symptoms, requiring glucocorticoids with or without cyclophosphamide as treatment.[
The patient did not report having an extremely painful (thunderclap) episode of headache, a finding more typically associated with RCVS than PACNS. However, the patient’s CSF did not exhibit pleocytosis, thereby bolstering the case for RCVS. In addition, a significant number of RBCs were found in the CSF, which is an expected finding in RCVS in the event of cSAH.[
Arachnoid cysts are mostly asymptomatic lesions but may present with neurological symptoms such as headache, nausea, and vision abnormalities. Suprasellar cysts may cause obstructive hydrocephalus through their compression of the third ventricle.[
Together these findings presented anatomic lesions that both potentially obstructed the flow of CSF and could explain the patient’s hydrocephalus. This hydrocephalus could result in symptoms of intermittent headache as well as visual changes especially in the setting of suprasellar mass effect. With these findings apparent on the repeat MRI and uncertainty as to the causative etiology of the patient’s symptoms, the decision was made to perform an ETV as treatment for the patient’s hydrocephalus. This procedure addressed both of the patient’s anatomic lesions by creating an alternative pathway for CSF flow. Following the surgery, the patient’s symptomatology resolved, and the patient was successfully discharged home.
Alternatively, a neurological surgeon may elect to insert a shunt to treat an arachnoid cyst. This treatment, however, may result in overdrainage of CSF caused by excessive shunting leading to intracranial hypotension and symptoms that include headache, nausea, dizziness, and seizures.[
CONCLUSION
A 57-year-old female who presented with progressive, severe headache, and visual changes was found to have obstructive hydrocephalus, aqueductal stenosis, RCVS, and a suprasellar arachnoid cyst following diagnostic work-up and ETV. Both RCVS and hydrocephalus can produce overlapping clinical syndromes. Initial assumptions regarding the importance of cSAH and multifocal vasospasm as causative etiologies for the patient’s symptoms biased the clinical decision-making away from focusing on hydrocephalus. This bias ultimately delayed the patient’s accurate diagnosis and treatment until additional testing challenged these initial assumptions of causality. This case report illustrates the importance of cognitive biases such as anchoring especially when dealing with complex medical diagnosis in the setting of multiple overlapping clinical syndromes. Clinicians must be prepared to reject their initial assumptions and change diagnostic course when such biases are suspected.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abdel Razek AA, Alvarez H, Bagg S, Refaat S, Castillo M. Imaging spectrum of CNS vasculitis. Radiographics. 2014. 34: 873-94
2. Birnbaum J, Hellmann DB. Primary angiitis of the central nervous system. Arch Neurol. 2009. 66: 704-9
3. Ducros A. Reversible cerebral vasoconstriction syndrome. Lancet Neurol. 2012. 11: 906-17
4. Ducros A, Boukobza M, Porcher R, Sarov M, Valade D, Bousser MG. The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients. Brain. 2007. 130: 3091-101
5. Ducros A, Fiedler U, Porcher R, Boukobza M, Stapf C, Bousser MG. Hemorrhagic manifestations of reversible cerebral vasoconstriction syndrome: Frequency, features, and risk factors. Stroke. 2010. 41: 2505-11
6. Glastonbury CM, Osborn AG, Salzman KL. Masses and malformations of the third ventricle: Normal anatomic relationships and differential diagnoses. Radiographics. 2011. 31: 1889-1905
7. Greenberg MS.editors. Handbook of Neurosurgery. New York: Thieme Medical Publishers; 2020. p. 262-5
8. Gui SB, Wang XS, Zong XY, Zhang YZ, Li CZ. Suprasellar cysts: Clinical presentation, surgical indications, and optimal surgical treatment. BMC Neurol. 2011. 11: 52
9. Gumus K, Dogan MS, Senol S, Doganay S. Flow void artifact mimicking aneurysm in the anterior communicating artery region on T1-and T2-weighted images. Quant Imaging Med Surg. 2015. 5: 633-4
10. Hall S, Smedley A, Sparrow O, Mathad N, Waters R, Chakraborty A. Natural history of intracranial arachnoid cysts. World Neurosurg. 2019. 126: e1315-20
11. Jallo GI, Kothbauer KF, Rectinos VM.editors. Handbook of Pediatric Neurosurgery. New York: Thieme Medical Publishers; 2018. p. 242-6
12. Miyajima M, Arai H, Okuda O, Hishii M, Nakanishi H, Sato K. Possible origin of suprasellar arachnoid cysts: neuroimaging and neurosurgical observations in nine cases. J Neurosurg. 2000. 93: 62-7
13. Mustansir F, Bashir S, Darbar A. Management of arachnoid cysts: A comprehensive review. Cureus. 2018. 10: e2458
14. Panagopoulos D, Karydakis P, Themistocleous M. Slit ventricle syndrome: Historical considerations, diagnosis, pathophysiology, and treatment review. Brain Circ. 2021. 7: 167-77
15. Rocha EA, Topcuoglu MA, Silva GS, Singhal AB. RCVS2 score and diagnostic approach for reversible cerebral vasoconstriction syndrome. Neurology. 2019. 92: e639-47
16. Ros B, Iglesias S, Linares J, Cerro L, Casado J, Arráez MA. Shunt overdrainage: Reappraisal of the syndrome and proposal for an integrative model. J Clin Med. 2021. 10: 3620
17. Strunk D, Schmidt-Pogoda A, Beuker C, Milles LS, Korsukewitz C, Meuth SG. Biomarkers in vasculitides of the nervous system. Front Neurol. 2019. 10: 591
18. Tisell M. How should primary aqueductal stenosis in adults be treated? A review. Acta Neurol Scand. 2005. 111: 145-53
19. Werring DJ. Reversible cerebral vasoconstriction syndrome and intracranial hemorrhage: Some answers, many questions. Stroke. 2010. 41: 2455-6