- Department of Neurosurgery, Ohnishi Neurological Center, Akashi, Hyogo Prefecture, Japan.
- Department of Neurosurgery, Nara Medical University, Kashihara, Nara Prefecture, Japan.
Correspondence Address:
Ryosuke Maeoka, Department of Neurosurgery, Nara Medical University, Kashihara, Nara Prefecture, Japan.
DOI:10.25259/SNI_825_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hiromu Sunada1, Ryosuke Maeoka1,2, Ichiro Nakagawa2, Hiroyuki Nakase2, Hideyuki Ohnishi1. A case of recurrent cavernous sinus dural arteriovenous fistula arising after superselective shunt occlusion and detected by venous arterial spin labeling. 08-Dec-2021;12:594
How to cite this URL: Hiromu Sunada1, Ryosuke Maeoka1,2, Ichiro Nakagawa2, Hiroyuki Nakase2, Hideyuki Ohnishi1. A case of recurrent cavernous sinus dural arteriovenous fistula arising after superselective shunt occlusion and detected by venous arterial spin labeling. 08-Dec-2021;12:594. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11279
Abstract
Background: Superselective shunt occlusion (SSSO) for cavernous sinus dural arteriovenous fistula (CSDAVF) avoids the risk of cranial nerve palsy, unlike entire sinus packing, but requires paying attention to recurrence. Distinguishing between true and paradoxical worsening of postoperative ophthalmic symptoms using a less-invasive modality is often difficult. Here, we report a case of true worsening of neuro-ophthalmic symptom by recurrent CSDAVF detected by venous-arterial spin labeling (ASL) on magnetic resonance imaging.
Case Description: A 55-year-old woman with neither contributory medical history nor previous head trauma presented with neuro-ophthalmic symptoms and pulsatile tinnitus. Digital subtraction angiography (DSA) revealed CSDAVF with multiple shunted pouches. She underwent successful transvenous SSSO, but neuroophthalmic symptom worsened after SSSO and venous-ASL revealed increased signal intensity in the right superior orbital vein (SOV). DSA confirmed recurrent CSDAVF and additional transvenous embolization was performed. Neuro-ophthalmic symptoms and venous-ASL hyperintensity on SOV improved postoperatively.
Conclusion: Venous-ASL is noninvasive and seems useful for detecting true worsening of neuro-ophthalmic symptoms of recurrent CSDAVF.
Keywords: Arterial spin labeling, Cavernous sinus dural arteriovenous fistula, Neuro-ophthalmic symptom, Superselective shunt occlusion
INTRODUCTION
Dural arteriovenous fistula (DAVF) is a cerebral vascular malformation characterized by direct communication between dural arteries and either a dural sinus or cortical vein.[
CASE DESCRIPTION
A 55-year-old woman with neither contributory medical history nor previous head trauma presented with bilateral chemosis, pulsatile tinnitus, exophthalmia, and 6th cranial nerve palsy. Magnetic resonance angiography (MRA) and time-of-flight (TOF) angiography suggested CSDAVF [
Video 1
Video 2
Video 3
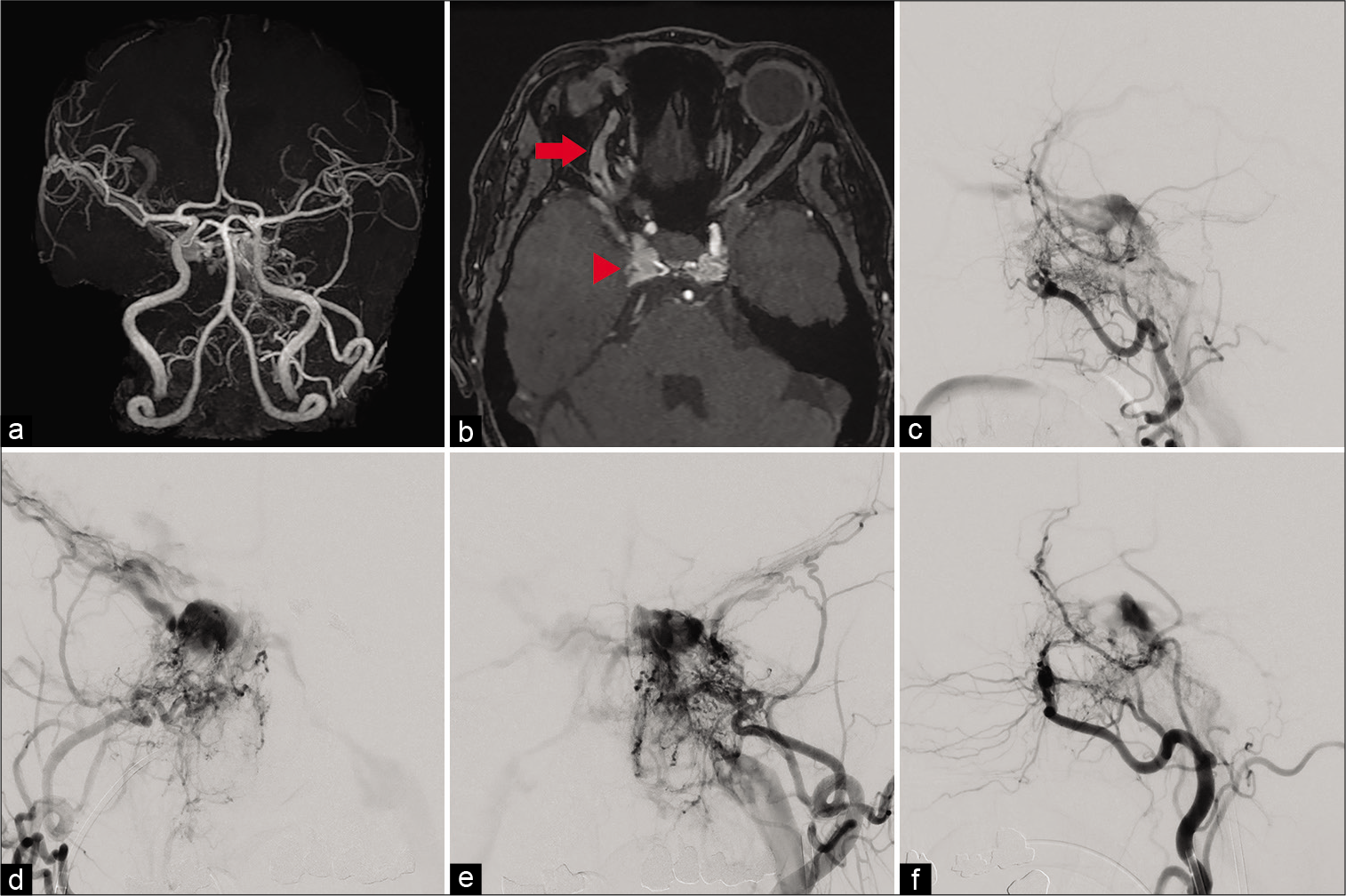
Figure 1:
(a) Magnetic resonance angiography suggests cavernous sinus dural arteriovenous fistula (CSDAVF). (b) Time-of-flight angiography suggests CSDAVF (red arrowhead) and dilation of the superior orbital vein (red arrow). (c) Lateral view of right external cerebral angiography reveals the CSDAVF fed by branches of the external carotid artery, has multiple shunted pouches located in the cavernous sinus, and drains into superior orbital vein (SOV), inferior petrosal sinus (IPS), and superficial middle cerebral veins (SMCVs). (d) Anteroposterior (AP) view of right external cerebral angiography reveals the CSDAVF fed by branches of the external carotid artery, has multiple shunted pouches located in the cavernous sinus, and drains into bilateral SOVs, IPSes, and SMCVs. (e) AP view of left external cerebral angiography reveals the CSDAVF fed by branches of the external carotid artery, has multiple shunted pouches located in bilateral compartments of the cavernous sinus, and drains into bilateral SOVs, IPSes, and SMCVs. (f) Lateral view of left external cerebral angiography reveals the CSDAVF fed by branches of the external carotid artery, has multiple shunted pouches located in the cavernous sinus, and drains into SOV, IPS, and SMCVs.
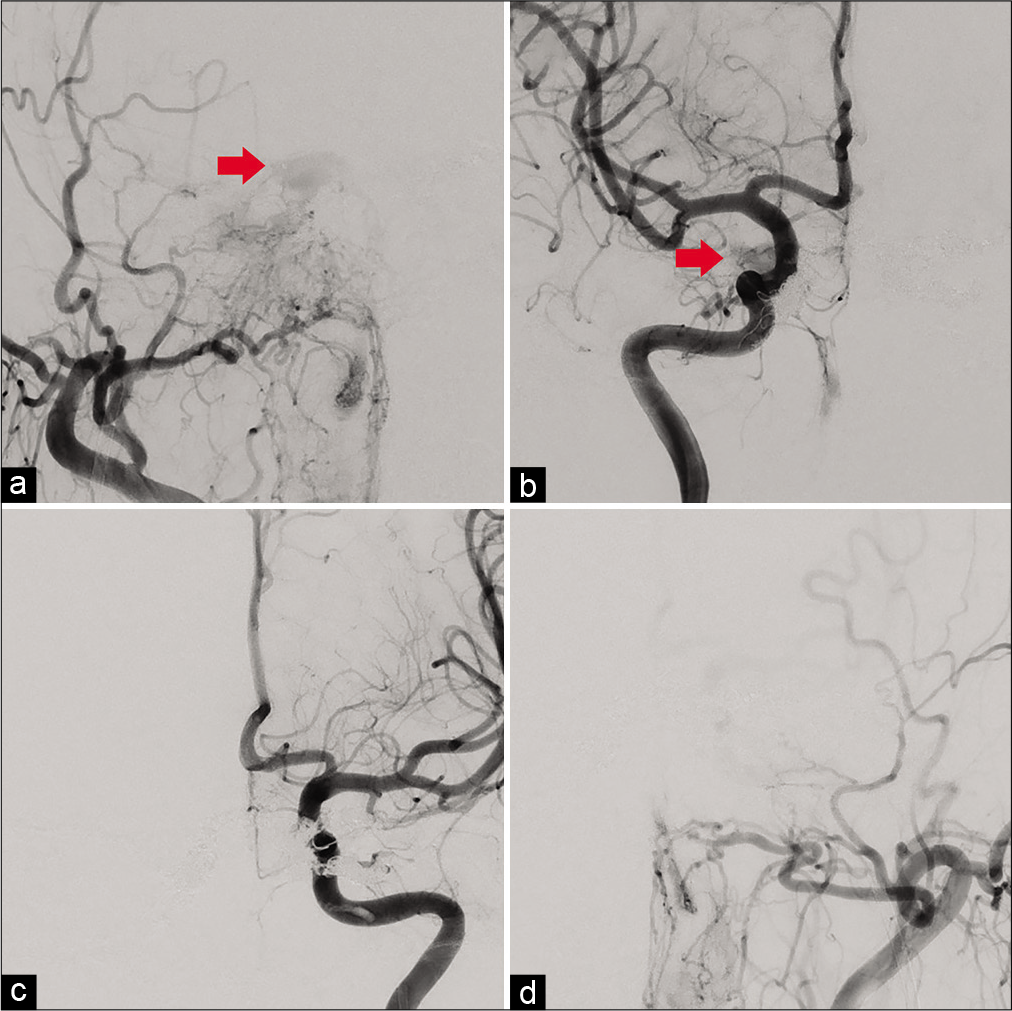
Figure 2:
(a) Anteroposterior (AP) view of right external cerebral angiography just after superselective shunt occlusion (SSSO) reveal the drainage route into the left superior orbital vein (SOV) has deteriorated, while that into the right SOV remains to a slight extent (red arrow). (b) AP view of right internal cerebral angiography just after SSSO reveal the drainage route into the left SOV has deteriorated, while that into the right SOV remains to a slight extent (red arrow). (c) AP view of left internal cerebral angiography reveal that the left feeders into the cavernous sinus dural arteriovenous fistula (CSDAVF) have been obliterated. (d) AP view of left external cerebral angiography reveal that the left feeders into the CSDAVF have been obliterated.
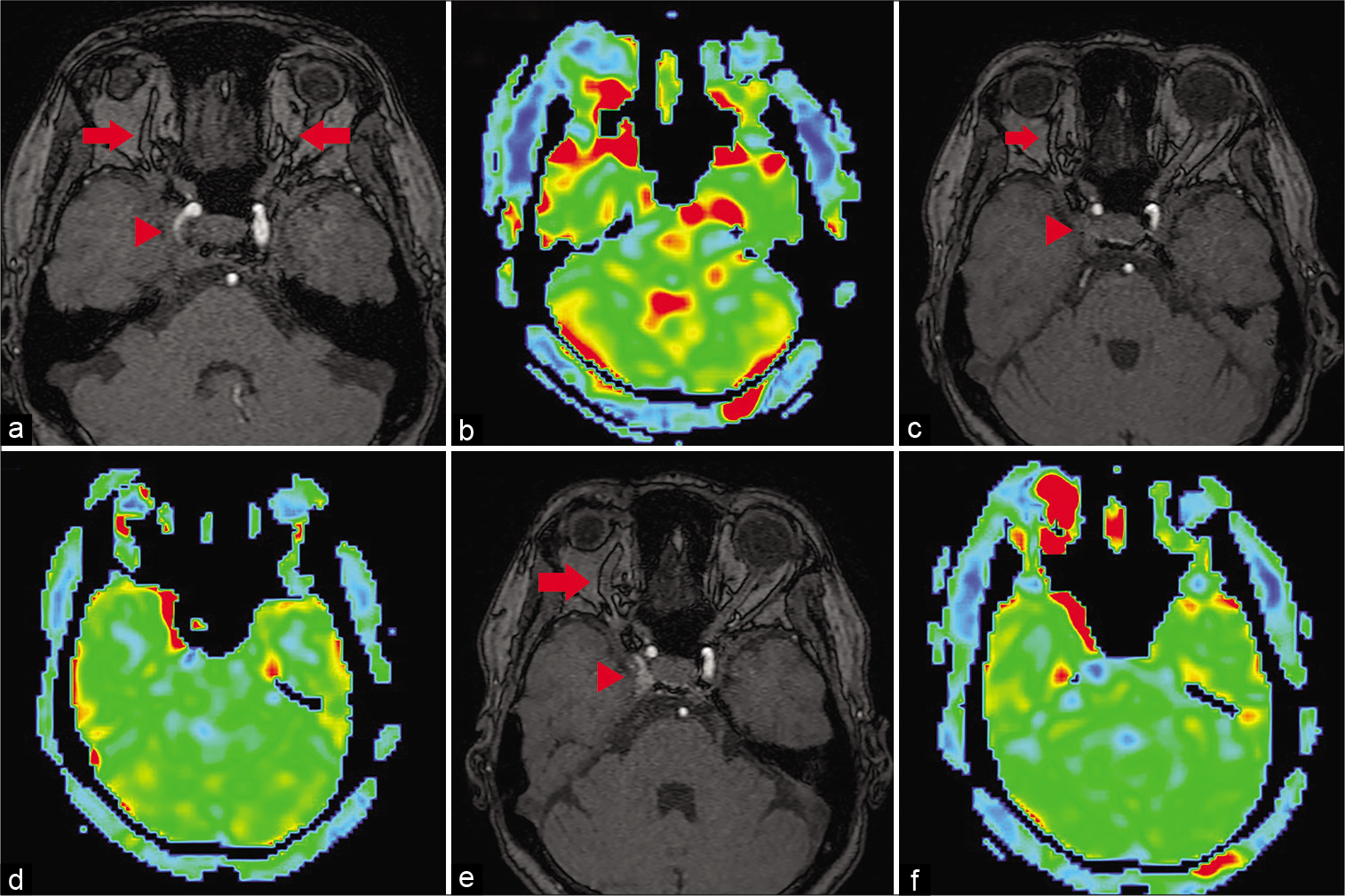
Figure 3:
(a) Time-of-flight (TOF) angiography just after first operation shows dilatation of bilateral superior orbital veins (SOVs) has improved (red arrow) and the cavernous sinus dural arteriovenous fistula (CSDAVF) has been obliterated (red arrowhead). (b) Venous-arterial spin labeling (ASL) confirms increased signal intensity at bilateral SOVs. (c) TOF angiography on the 1 month later from superselective shunt occlusion (SSSO) shows dilatation of the SOVs (red arrow) and obliteration of the CSDAVF (red arrowhead). (d) Venous-ASL on the 1 month later from SSSO reveals resolution of increased signal intensity in the left SOV, but slight remnant hyperintensity of the right SOV. (e) TOF angiography on the 2 months later from SSSO shows dilatation of only the right SOV (red arrow) and confirmation of hyperintense signal in the right CS (red arrowhead). (f) Venous-ASL on the 2 months later from SSSO shows exacerbation of signal hyperintensity in the right SOV.
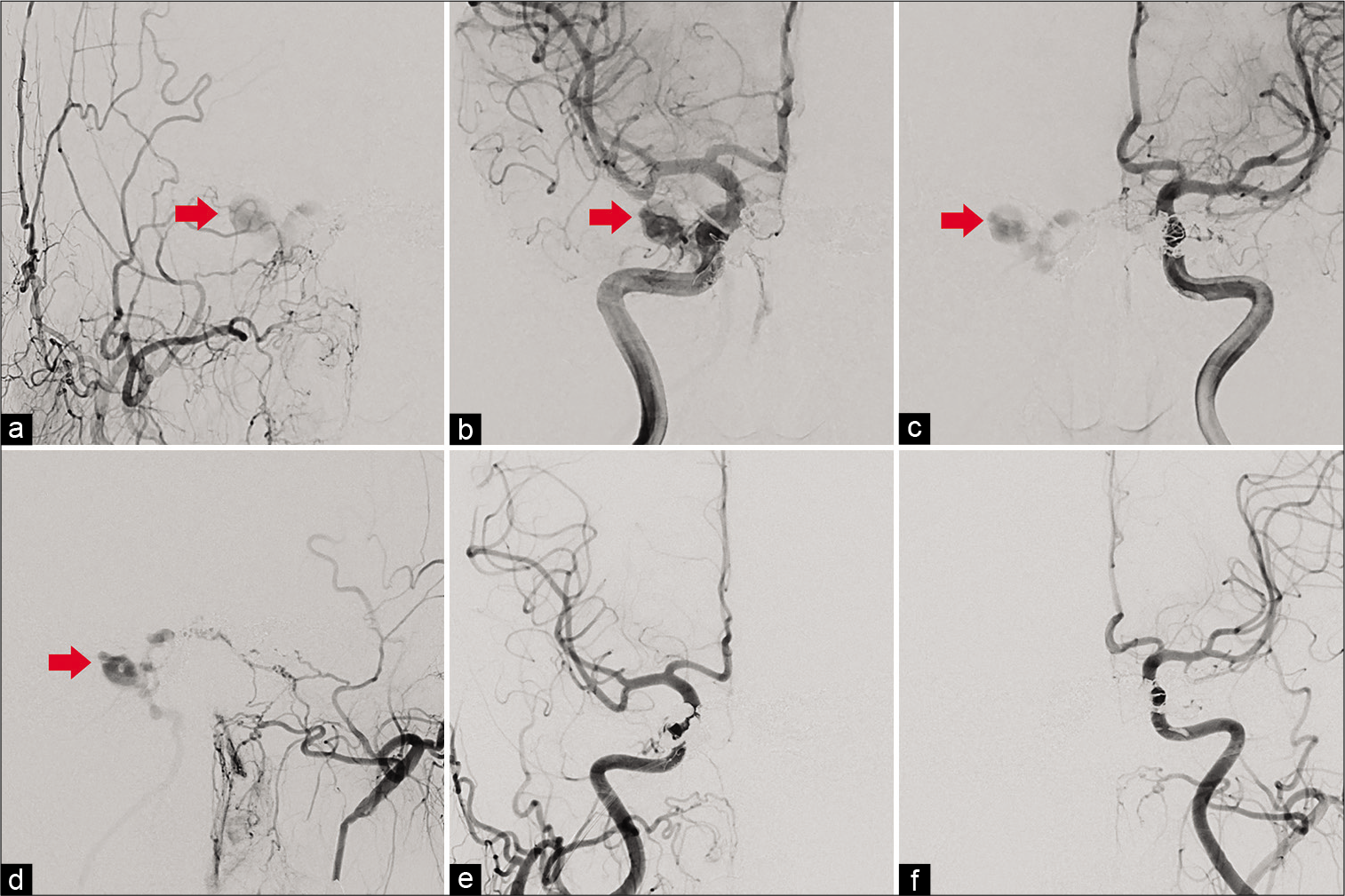
Figure 4:
(a) Right external cerebral angiography demonstrate recurrence of cavernous sinus dural arteriovenous fistula (CSDAVF) draining into the right superior orbital vein (SOV) (red arrow). (b) Right internal cerebral angiography demonstrate recurrence of CSDAVF draining into the right SOV (red arrow). (c) Left internal cerebral angiography also demonstrate recurrence of CSDAVF draining into the right SOV (red arrow). (d) Left external cerebral angiography also demonstrate recurrence of CSDAVF draining into the right SOV (red arrow). (e) Postoperative right cerebral angiography reveals complete obliteration of the CSDAVF. (f) Postoperative left cerebral angiography reveals complete obliteration of the CSDAVF.
Video 4
DISCUSSION
Neuro-ophthalmic symptoms caused by venous reflux into the SOV are common symptoms of CSDAVF and also occur in natural course or after endovascular treatment. The syndrome of paradoxical worsening of neuro-ophthalmic symptoms after endovascular treatment has been attributed to thrombosis of the SOV, the CS, and their tributaries with improvement in the clinical findings of the CSDAVF.[
We recognize that venous-ASL has some drawbacks. First, artifacts may be present in the image. False-positives and false-negatives can occur, and the sensitivity of venousASL is inferior to that of DSA. Second, ASL offered limited spatial resolution and the classification of DAVF could not be determined from ASL alone. Definitive diagnosis of DAVF thus requires DSA, as does planning of the treatment strategy.
CONCLUSION
We have reported a case of recurrent CSDAVF after treatment with SSSO, detected by venous-ASL on MRI. Venous-ASL offers high sensitivity and specificity for the presence of DAVF and seems useful for completely noninvasive differentiation between true and paradoxical worsening of neuro-ophthalmic symptoms of CSDAVF.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos available on:
References
1. Agid R, Willinsky RA, Haw C, Souza MP, Vanek IJ, terBrugge KG. Targeted compartmental embolization of cavernous sinus dural arteriovenous fistulae using transfemoral medial and lateral facial vein approaches. Neuroradiology. 2004. 46: 156-60
2. Amukotuwa SA, Marks MP, Zaharchuk G, Calamante F, Bammer R, Fischbein N. Arterial spin-labeling improves detection of intracranial dural arteriovenous fistulas with MRI. AJNR Am J Neuroradiol. 2018. 39: 669-77
3. Cloft HJ, Joseph CJ, Dion JE. Risk of cerebral angiography in patients with subarachnoid hemorrhage, cerebral aneurysm, and arteriovenous malformation a meta-analysis. Stroke. 1999. 30: 317-20
4. Cognard C, Gobin YP, Pierot L, Bailly AL, Houdart E, Casasco A. Cerebral dural arteriovenous fistulas-clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995. 194: 671-80
5. Deibler AR, Pollock JM, Kraft RA, Tan H, Burdette JH, Maldjian JA. Arterial spin-labeling in routine clinical practice, Part 1: Technique and artifacts. AJNR Am J Neuroradiol. 2008. 29: 1228-34
6. Deibler AR, Pollock JM, Kraft RA, Tan H, Burdette JH, Maldjian JA. Arterial spin-labeling in routine clinical practice, Part 3: Hyperperfusion patterns. AJNR Am J Neuroradiol. 2008. 29: 1428-35
7. Farb RI, Agid R, Willinsky RA, Johnstone DM, Terbrugge KG. Cranial dural arteriovenous fistula: Diagnosis and classification with time-resolved MR angiography at 3T. AJNR Am J Neuroradiol. 2009. 30: 1546-51
8. Hirabuki N, Mitomo M, Miura T, Hashimoto T, Kawai R, Kozuka T. External carotid artery embolization of dural arteriovenous malformations involving the cavernous sinus. Outcome and role of venous thrombosis. Acta Radiol. 1990. 31: 197-201
9. Iwamura M, Midorikawa H, Shibutani K, Kakuta A, Maruyama S, Yotsuya C. High-signal venous sinuses on MR angiography: Discrimination between reversal of venous flow and arteriovenous shunting using arterial spin labeling. Neuroradiology. 2021. 63: 889-96
10. Le TT, Fischbein NJ, Andre JB, Wijman C, Rosenberg J, Zaharchuk G. Identification of venous signal on arterial spin labeling improves diagnosis of dural arteriovenous fistulas and small arteriovenous malformations. AJNR Am J Neuroradiol. 2012. 33: 61-8
11. Piske RL, Campos CM, Chaves JB, Abicalaf R, Dabus G, Batista LL. Dural sinus compartment in dural arteriovenous shunts-a new angioarchitectural feature allowing superselective transvenous dural sinus occlusion treatment. Am J Neuroradiol. 2005. 26: 1715-22
12. Sato M, Izumi T, Matsubara N, Nishihori M, Miyachi S, Wakabayashi T. Evaluation for shunted pouches of cavernous sinus dural arteriovenous fistula and the treatment outcome of transvenous embolization. Interv Neuroradiol. 2018. 24: 189-96
13. Satow T, Murao K, Matsushige T, Fukuda K, Miyamoto S, Iihara K. Superselective shunt occlusion for the treatment of cavernous sinus dural arteriovenous fistulae. Neurosurgery. 2013. 73: ons100-5
14. Sergott RC, Grossman RI, Savino PJ, Bosley TM, Schatz NJ. The syndrome of paradoxical worsening of dural-cavernous sinus arteriovenous malformations. Opthalmology. 1987. 94: 205-12
15. Willems PW, Brouwer PA, Barfett JJ, terBrugge KG, Krings T. Detection and classification of cranial dural arteriovenous fistulas using 4D-CT angiography: Initial experience. AJNR Am J Neuroradiol. 2011. 32: 49-53