- Department of Neurosurgery, Ashikaga Red Cross Hospital, Ashikaga, Japan.
- Department of Internal Medicine, Ashikaga Red Cross Hospital, Ashikaga, Japan.
Correspondence Address:
Shunsuke Shibao, Department of Neurosurgery, Ashikaga Red Cross Hospital, Ashikaga, Japan.
DOI:10.25259/SNI_780_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Shunsuke Shibao1, Makoto Kaburaki2, Katsuya Saito1, Hideyuki Tomita1. A case of subarachnoid and intracerebral hemorrhages complicated by trichosporonosis. 14-Oct-2022;13:472
How to cite this URL: Shunsuke Shibao1, Makoto Kaburaki2, Katsuya Saito1, Hideyuki Tomita1. A case of subarachnoid and intracerebral hemorrhages complicated by trichosporonosis. 14-Oct-2022;13:472. Available from: https://surgicalneurologyint.com/surgicalint-articles/11927/
Abstract
Background: Trichosporonosis has an extremely poor prognosis. In this report, we describe a case of subarachnoid hemorrhage and intracerebral hemorrhage due to a fungal aneurysm caused by Trichosporon.
Case Description: A 71-year-old woman who experienced subcortical hemorrhage developed a subarachnoid hemorrhage. Endovascular parent artery occlusion was performed for a fungal aneurysm in the left posterior cerebral artery caused by Trichosporon. After surgery, voriconazole and liposomal amphotericin B were administered. The patient died of massive left putamen hemorrhage.
Conclusion: Effective treatment for intracranial hemorrhage due to trichosporonosis has not yet been established and an accumulation of cases is required.
Keywords: Fungal aneurysm, Intracerebral hemorrhage, Subarachnoid hemorrhage, Trichosporonosis
INTRODUCTION
Trichosporon is an opportunistically infective yeast-like fungus. About 74.7% of Trichosporon infections reach the bloodstream, which is associated with an extremely poor prognosis.[
We describe a case of subarachnoid hemorrhage due to rupture of a fungal aneurysm caused by Trichosporon, resulting in recurrent intracerebral hemorrhage and death.
CLINICAL SUMMARY
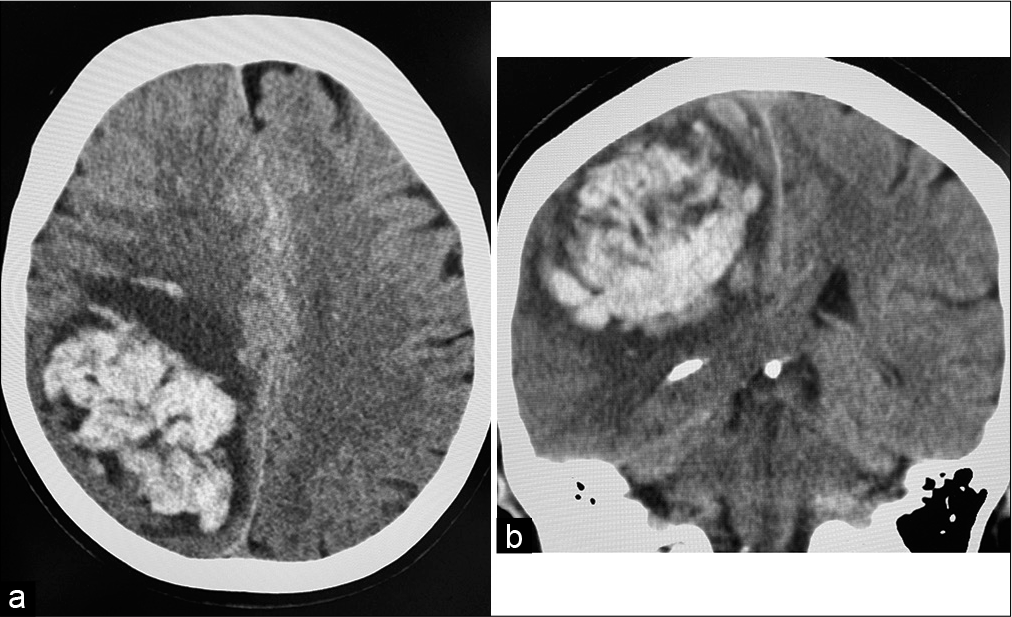
The patient is a 71-year-old woman who underwent surgical aortic mechanical valve replacement for aortic dissection and was started on warfarin postoperatively. Seven months after her aortic valve replacement surgery, she experienced her first intracerebral hemorrhage. No other underlying medical conditions, such as diabetes or immunodeficiencies, were noted. The hemorrhage was subcortical, in the right parietal lobe. The hematoma was removed by craniotomy in the neurosurgery department of another hospital (day 0) [
Thirty-eight days after the onset of the first hemorrhage (day 38), the patient was transferred to our hospital for convalescent rehabilitation. At that time, neurological findings included a Japanese Coma Scale 1 and GCS 15 (E4 V5 M6) level of consciousness, left unilateral spatial neglect, left facial paralysis, and left hemiplegia. Thirteen days after transfer (day 51), the patient developed a fever of unknown etiology, and antibiotic therapy (levofloxacin, ceftriaxone, and meropenem) was administered for possible urinary tract infection and meningitis. The patient’s laboratory results during the course of the fever included white blood cell count 11600/ul, C-reactive protein 3.76 mg/L, procalcitonin 0.18 ng/mg, and βD glucan 7.2 pg/ml. Cerebrospinal fluid examination revealed a cell count of 46.2/µL (57 mononuclear cells and 43 polymorphonuclear cells), 70 mg/dL protein, and 45 mg/dL sugar. Echocardiography showed an echo-free around the prosthetic valve, but coronary computed tomography (CT) showed no obvious abscess formation around the valve.
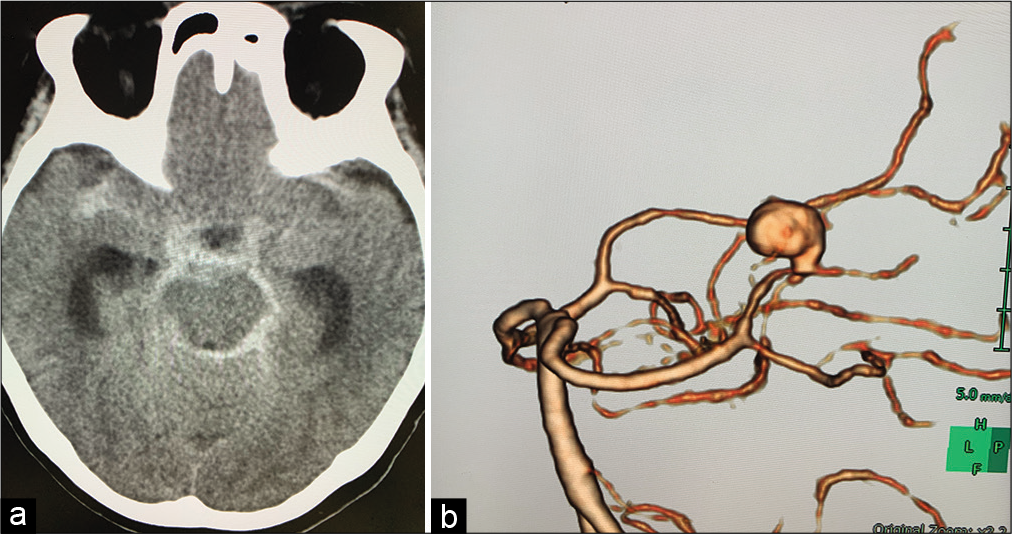
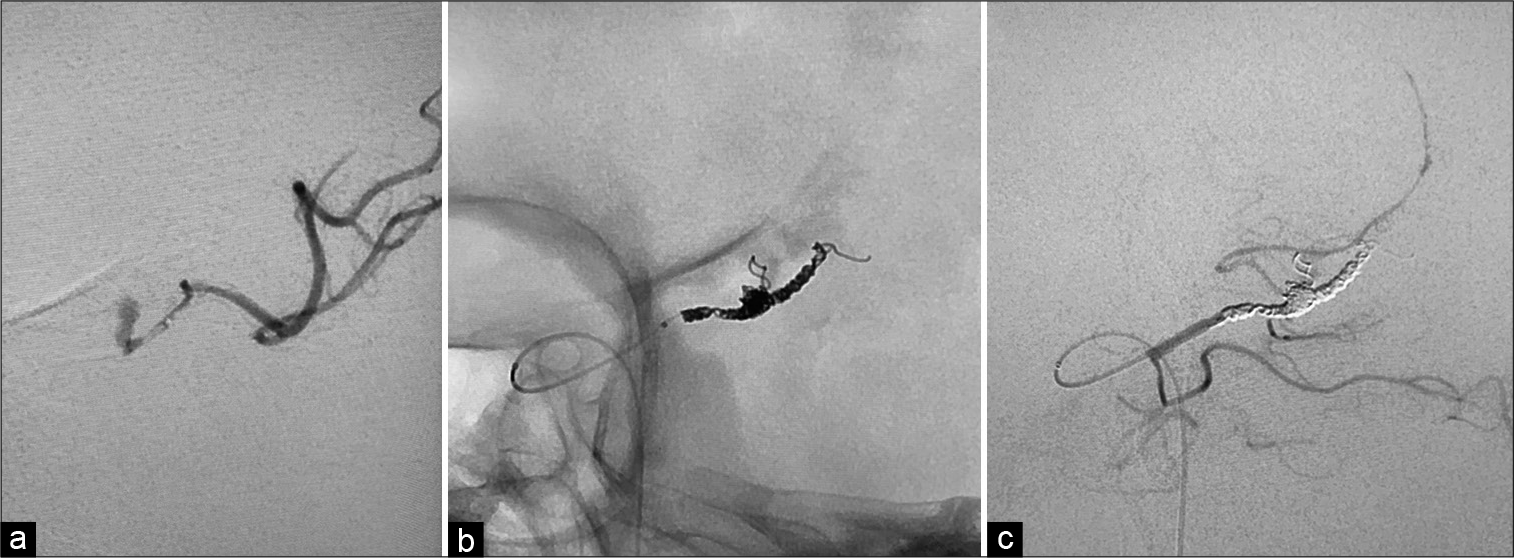
Three days later (day 54), the patient developed subarachnoid hemorrhage and was referred to our department [
Postoperatively, the patient was treated for vasospasm and started on voriconazole (VRCZ) as an antifungal. On the 3rd postoperative day (day 57), head CT showed asymptomatic subcortical hemorrhage in the left temporal lobe [
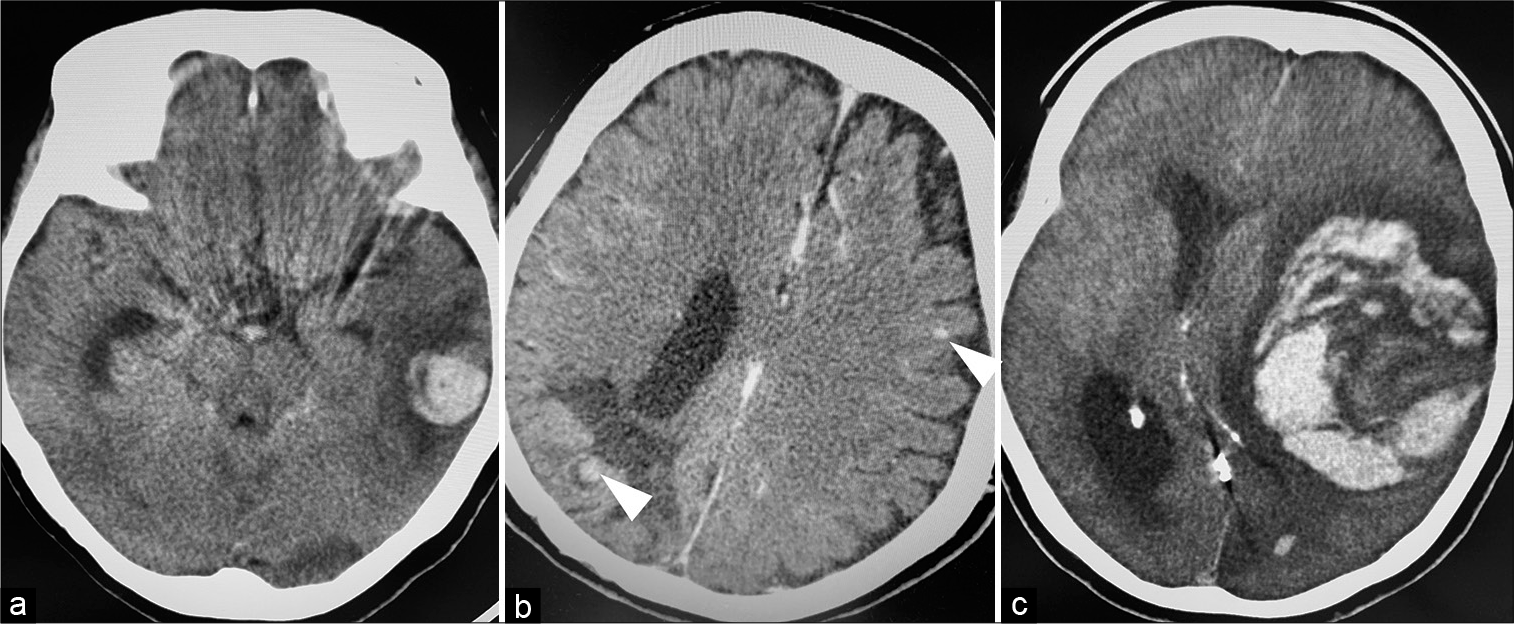
Figure 4:
Time course of head CT scan after endovascular parent artery occlusion. On postoperative day 3 (day 57), head CT showed left temporal lobe subcortical hemorrhage (a) on day 85, head contrast-enhanced CT showed multiple microaneurysms (arrow heads) (b) on day 92, head CT showed left putamen hemorrhage with cerebral herniation (c).
DISCUSSION
Trichosporon species are present in the human microbiota of skin and gastrointestinal tract.[
As for fungal aneurysms, the known routes of entry include hematogenous dissemination, direct invasion, and surgical contamination. The most frequently reported fungi are Aspergillus, Candida, Zygomycetes, and Coccidiodes. There are no reports of intracranial aneurysms caused by Trichosporon. Aneurysms caused by direct invasion are more common in proximal arteries, such as the internal carotid artery, while those caused by blood dissemination are more common in distal arteries, such as the anterior cerebral artery, middle cerebral artery, and PCA. Reports of both craniotomy and endovascular surgery indicate that the location and shape of the aneurysm determine which is more appropriate for management. Endovascular parent vessel occlusion may be indicated for distally infected aneurysms.[
An accumulation of reports is needed to formulate a proper plan of management in such cases. Furthermore, the clinical summary may aid clinicians in the diagnosis and treatment of similar rare occurrences of trichosporonosis infection.
CONCLUSION
We report an unprecedented case of intracranial fungal aneurysm caused by trichosporonosis. Effective intracranial trichosporonosis management has not yet been established and the treatment for such cases with subarachnoid or intracerebral hemorrhage requires careful analysis of multiple cases.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Basiri K, Meidani M, Rezaie F, Soheilnader S, Fatehi F. A rare case of Trichosporon brain abscess, successfully treated with surgical excision and antifungal agents. Neurol Neurochir Pol. 2012. 46: 92-5
2. Castano G, Yarrarapu SN, Mada PK, editors. Trichosporonosis. Treasure Island (FL): StatPearls; 2021. p.
3. Champeaux C, Walker N, Derwin J, Grivas A. Successful delayed coiling of a ruptured growing distal posterior cerebral artery mycotic aneurysm. Neurochirurgie. 2017. 63: 17-20
4. Clare CE, Barrow DL. Infectious intracranial aneurysms. Neurosurg Clin N Am. 1992. 3: 551-66
5. Hajjar J, Restrepo A, Javeri H, Wiederhold NP, Papanastassiou AM, Patterson TF. Multiple brain abscesses caused by Trichosporon inkin in a patient with x-linked chronic granulomatous disease (CGD) successfully treated with antifungal therapy. J Clin Immunol. 2017. 37: 519-23
6. Heslop OD, Nyi MP, Abbott SP, Rainford LE, Castle DM, Coard KC. Disseminated trichosporonosis in a burn patient: Meningitis and cerebral abscess due to Trichosporon asahii. J Clin Microbiol. 2011. 49: 4405-8
7. Kamei S, Takasu T. Nationwide survey of the annual prevalence of viral and other neurological infections in Japanese inpatients. Intern Med. 2000. 39: 894-900
8. Kumar A, Udayakumaran S, Babu R, Rajamma BM, Prakash A, Panikar D. Trichosporon asahii infection presenting as chronic meningo-ventriculitis and intra ventricular fungal ball: A case report and literature review. Mycoses. 2015. 58: 99-103
9. Kushima H, Tokimatsu I, Ishii H, Kadota J. Antifungal susceptibility and drug-resistant mechanism of Trichosporon. Med Mycol J. 2015. 56: J123-8
10. Mathews MS, Prabhakar S. Chronic meningitis caused by Trichosporon beigelii in India. Mycoses. 1995. 38: 125-6
11. Milan EP, Silva-Rocha WP, De Almeida JJ, Fernandes TU, Prudente AL, De Azevedo MF. Trichosporon inkin meningitis in Northeast Brazil: First case report and review of the literature. BMC Infect Dis. 2018. 18: 470
12. Rastogi VL, Nirwan PS. Invasive trichosporonosis due to Trichosporon asahii in a non-immunocompromised host: A rare case report. Indian J Med Microbiol. 2007. 25: 59-61
13. Ruan SY, Chien JY, Hsueh PR. Invasive trichosporonosis caused by Trichosporon asahii and other unusual Trichosporon species at a medical center in Taiwan. Clin Infect Dis. 2009. 49: e11-7
14. Surmont I, Vergauwen B, Marcelis L, Verbist L, Verhoef G, Boogaerts M. First report of chronic meningitis caused by Trichosporon beigelii. Eur J Clin Microbiol Infect Dis. 1990. 9: 226-9
15. Thien SY, Chung SJ, Tan AL, Hwang WY, Tan BH, Tan TT. Recurrent trichosporonosis with central nervous system involvement in an allogeneic hematopoietic stem cell transplant recipient. Transpl Infect Dis. 2016. 18: 768-72
16. Walsh TJ. Trichosporonosis. Infect Dis Clin North Am. 1989. 3: 43-52
17. Yamaguchi J, Kawabata T, Motomura A, Hatano N, Seki Y. Fungal internal carotid artery aneurysm treated by trapping and high-flow bypass: A case report and literature review. Neurol Med Chir (Tokyo). 2016. 56: 89-94