- Department of Neurosurgery, Nagoya Ekisaikai Hospital,
- Department of Neurosurgery, JCHO Chukyo Hospital, Nagoya, Japan.
Correspondence Address:
Yusuke Sakamoto, Department of Neurosurgery, Nagoya Ekisaikai Hospital, Nagoya, Japan.
DOI:10.25259/SNI_415_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Yusuke Sakamoto1, Kenko Maeda2, Masaya Takemoto2, Jungsu Choo2, Mizuka Ikezawa2, Ohju Fujita2, Fumihiro Sago2, Daiki Somiya2, Akira Ikeda2. A case of symptomatic carotid artery occlusion after aortic arch replacement treated with carotid-carotid crossover bypass. 23-Jun-2022;13:273
How to cite this URL: Yusuke Sakamoto1, Kenko Maeda2, Masaya Takemoto2, Jungsu Choo2, Mizuka Ikezawa2, Ohju Fujita2, Fumihiro Sago2, Daiki Somiya2, Akira Ikeda2. A case of symptomatic carotid artery occlusion after aortic arch replacement treated with carotid-carotid crossover bypass. 23-Jun-2022;13:273. Available from: https://surgicalneurologyint.com/surgicalint-articles/11680/
Abstract
Background: Symptomatic common carotid artery (CCA) occlusion is rare and its treatment remains unestablished. Although cases of subclavian-to-carotid bypass have been reported, very few cases of carotid-tocarotid crossover bypass have been reported, despite its advantages. We report a case of Riles type 1A symptomatic CCA occlusion after aortic arch replacement that was treated with carotid-to-carotid crossover bypass with favorable outcomes.
Case Description: A 65-year-old woman with a history of hypertension, hyperlipidemia, diabetes, and total arch replacement for thoracic aortic aneurysm was admitted to our hospital with a complaint of the right hemiparesis and motor aphasia. Head magnetic resonance imaging revealed a fresh infarction in the left cerebral hemisphere. Cervical computed tomography (CT) angiography revealed left CCA occlusion. Thoracic CT angiography showed severe stenosis of the left subclavian artery. SPECT showed a general decrease in blood flow in the left cerebral hemisphere. We performed a carotid-to-carotid crossover bypass with a synthetic graft that was passed through the subcutaneous tunnel. First, the right carotid artery-synthetic graft end-to-side anastomosis was performed. Subsequently, we performed synthetic graft-left CCA end-to-side anastomosis. The postoperative course was uneventful. Cervical computed tomography angiography showed perfect patency of the crossover bypass. The patient recovered almost completely and was independently performing daily activities.
Conclusion: Carotid-to-carotid crossover bypass is a durable treatment for symptomatic CCA occlusion. Further studies are needed to compare its outcomes with those of other methods and to confirm our findings with larger sample size.
Keywords: Aortic arch replacement, Common carotid artery occlusion, Crossover bypass, Stroke, Synthetic graft
INTRODUCTION
Compared to symptomatic internal carotid artery (ICA) occlusion, symptomatic common carotid artery (CCA) occlusion is a relatively rare disease.[
CASE DESCRIPTION
The authors obtained written informed consent from the patient. No approval from the IRB was sought as this article is a case report.
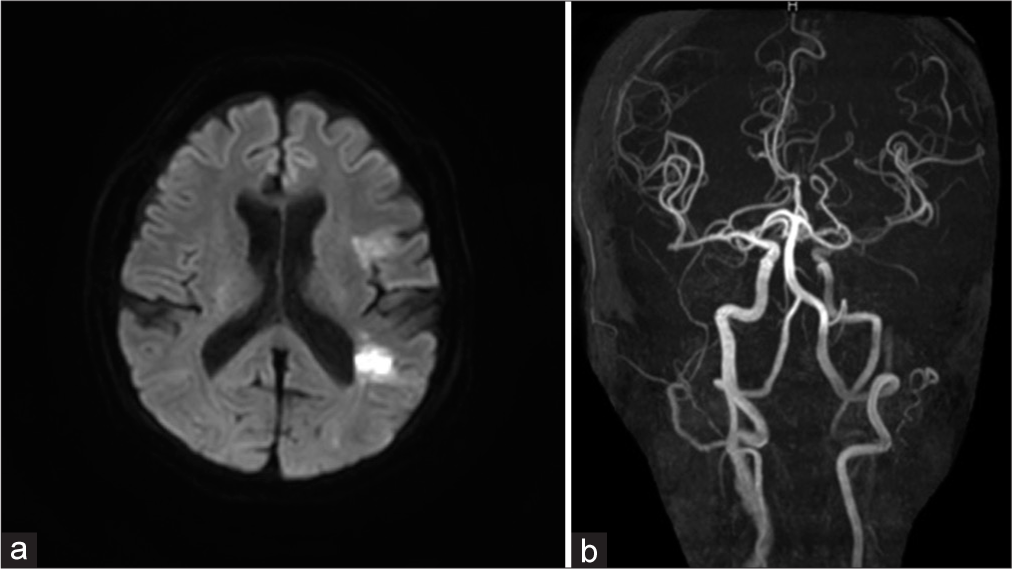
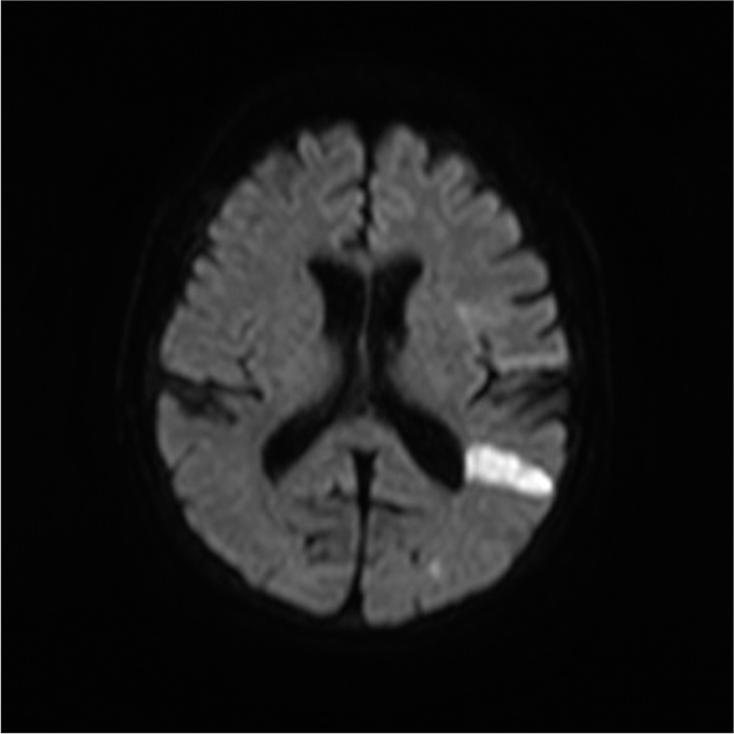
The patient was a 65-year-old woman with a history of hypertension, hyperlipidemia, diabetes, atherosclerotic occlusion of the right subclavian artery and right femoral artery, and thoracic aortic aneurysm. She was admitted to our hospital with a complaint of the right hemiparesis (manual muscle testing [MMT] = 2) and motor aphasia. The Glasgow Coma Scale score was E4V3M6 on arrival. Diffusion-weighted magnetic resonance imaging (MRI) of the head showed a high-intensity zone in the left insular cortex and temporal lobe [
Figure 2:
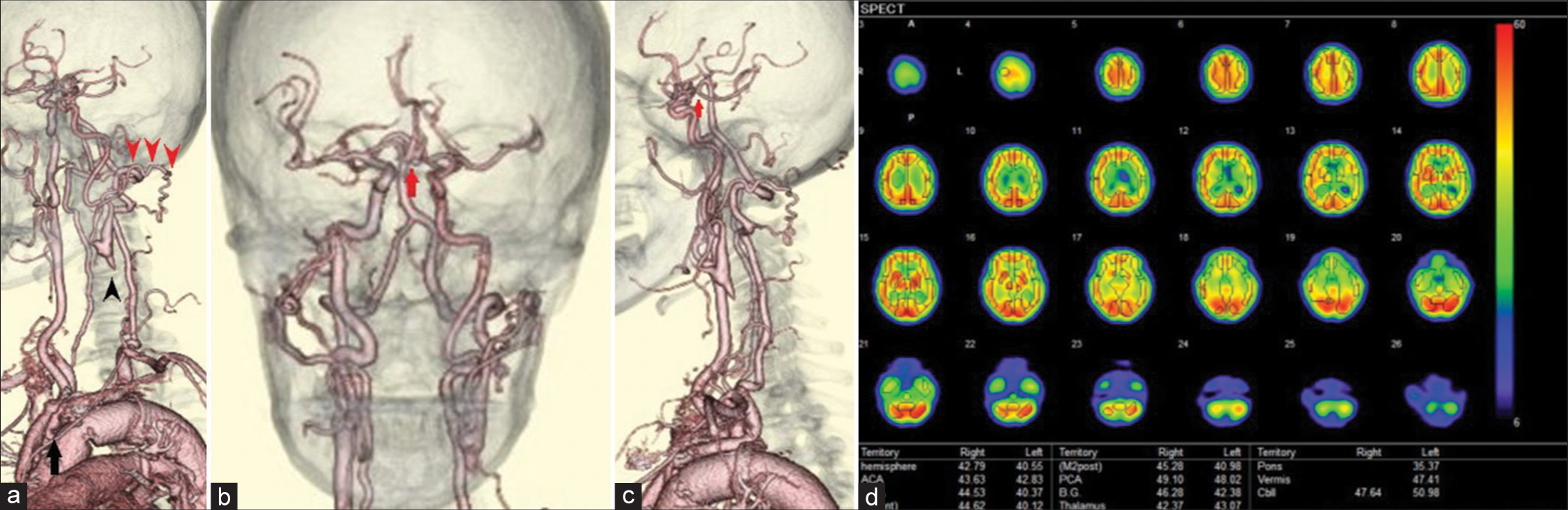
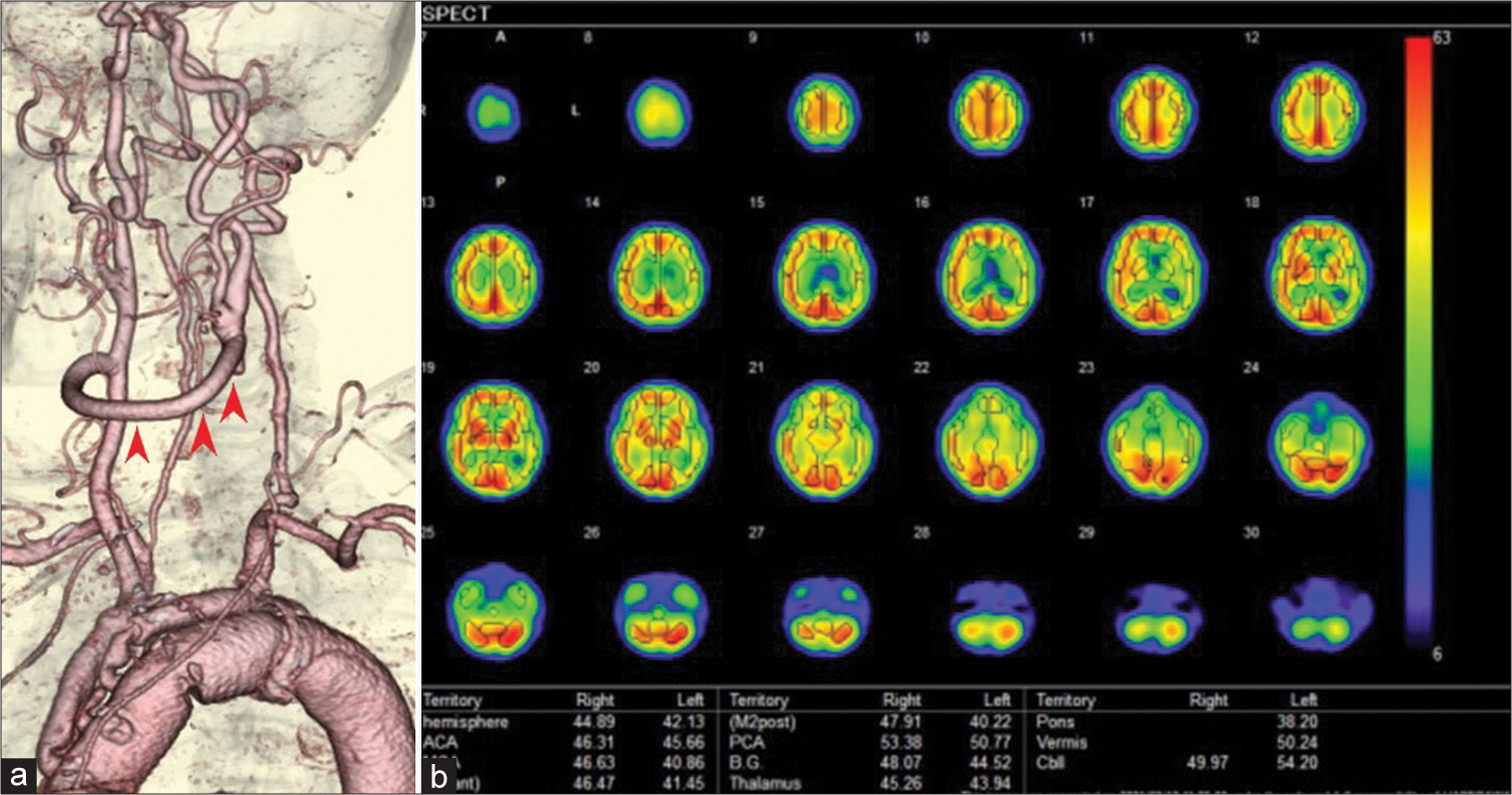
(a) The left cervical computed tomography angiography (CTA), left oblique view. The left common carotid artery was totally occluded from its origin to just before the carotid bifurcation (black arrowheads). Collateral anastomosis between the left occipital artery and a small branch of the left vertebral artery was detected (red arrowheads). Severe stenosis of the left subclavian artery was also observed (black arrow). (b) Head-and-neck CTA, front view. The anterior communicating artery was well developed (arrow), although there was a small unruptured aneurysm in the artery. (c) The left cervical CTA, left lateral view. The left posterior communicating artery was hypoplastic and not visible on CTA (arrow). (d) Single-photon emission computed tomography showed a general decrease in blood flow in the left cerebral hemisphere at rest.
Surgical strategy
Due to severe stenosis of the left subclavian artery, left subclavian- or axillary-to-carotid bypass could not be performed. We consulted our endovascular surgeon regarding the risk of performing transcatheter angioplasty. There was a fear of catheter-induced suture injury of the aorta synthetic graft, which could be catastrophic if performed on this patient. Furthermore, we wished to avoid a complicated two-stage vascular reconstruction, which would involve subclavian to carotid bypass following subclavian angioplasty, to reduce invasiveness. Although we considered a bonnet bypass, the left STA was too thin to allow sufficient blood flow. In addition, the patient had unruptured aneurysms in the right MCA and anterior communicating artery. We avoided the bonnet bypass in case surgical clipping would be needed for aneurysms in the future. The right CCA with intermediate stenosis was the only choice for the donor site. The patient had a history of arteriosclerotic occlusion of the femoral artery and right subclavian artery and had undergone vascular reconstruction. We were not certain of the condition of the radial artery graft. Therefore, we decided to perform a carotid-to-carotid crossover bypass with a synthetic vascular graft.
Surgery
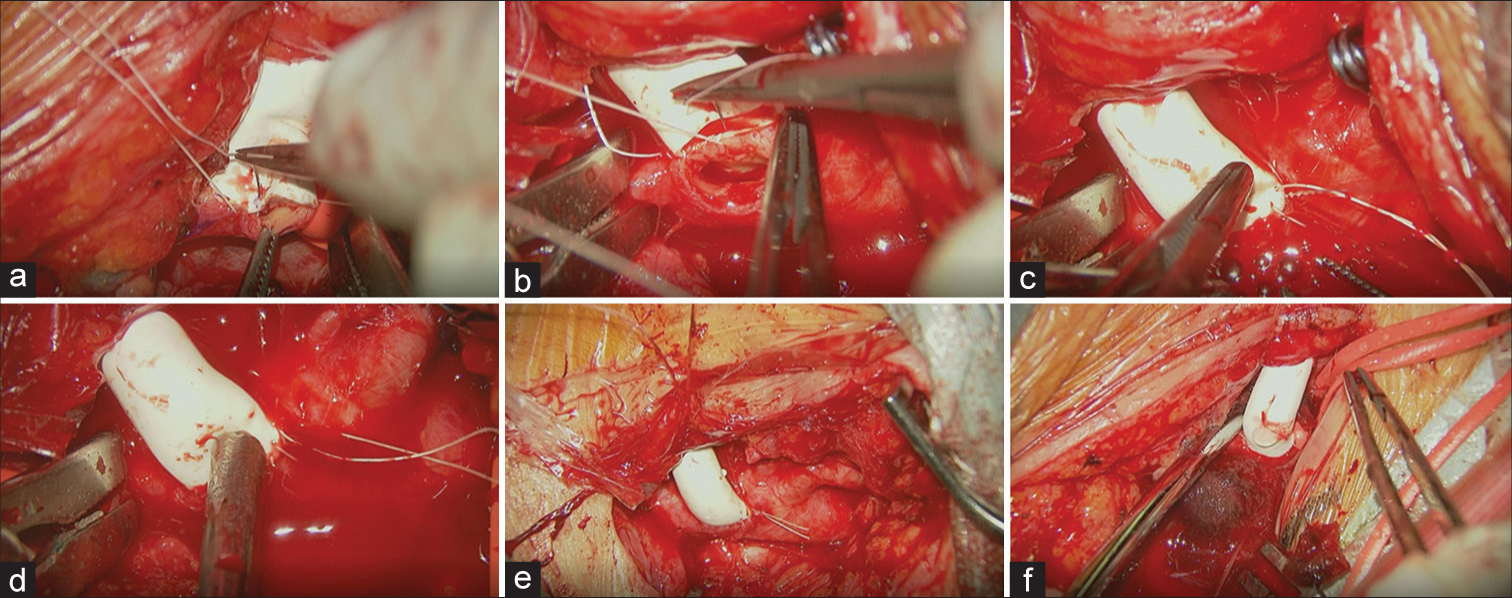
On day 25, vascular reconstructive surgery was performed. Dual antiplatelet therapy (aspirin 200 mg/day and clopidogrel 75 mg/day) was administered until 7 days before the surgery and continued with a single antiplatelet drug (aspirin 200 mg/day) until surgery. The patient was placed in a supine position under general anesthesia. The somatosensory evoked potential (SEP), motor evoked potential (MEP), and near-infrared spectroscope (NIRS) were monitored. First, the neck was rotated to the left side at approximately 30°. A linear skin incision along the sternocleidomastoid muscle (SCM) was made to expose the right CCA. After exposing 3 cm of the right CCA, we began the contralateral procedure. The neck was rotated to the right side at approximately 30°, and a linear skin incision was made along the left SCM. We then exposed the left CCA, ECA, and ICA. Indocyanine green showed a stump of an isolated CCA. The length of the stump was approximately 10 mm. Based on the length of the stump and the diameter of the CCA, we chose a 6 mm Gore-Tex® Stretch vascular graft and CV-7 Gore-Tex suture® (W.L. Gore and Associates, Inc., Medical Product Division, Flagstaff, AZ, USA). We created a subcutaneous tunnel with a shunt passer and passed it through a synthetic graft. Before clamping, edaravone 30 mg, methylprednisolone (125 mg), phenytoin (250 mg), and 20% mannitol (300 ml) were administered for cerebral protection. Heparin 4000 U was also infused. We then clamped the right CCA and made a linear vascular incision with a spits scalpel and dilated the arteriotomy with an aortic puncher. Since the orifice of the carotid artery was very small, the carotid shunt, which may have interrupted the back wall suture, was not used. We then performed right carotid artery-synthetic graft end-to-side anastomosis with a running suture [
Figure 4:
(a) The right common carotid artery was exposed and an end-to-side anastomosis with the synthetic graft was performed. (b) The left common carotid artery was exposed, and an end-to-side anastomosis with the synthetic graft was performed. Note that a double-armed suture thread was used, and a staying suture was only made on the heel (proximal side) of the orifice. (c) Before completing the ligation of the double-armed thread, the left internal carotid artery (ICA) was temporarily released to flush the air and debris. Note that the bubble of air was flushed from the toe (distal side) of the orifice of the synthetic graft anastomosed site with retrograde blood flow from the left ICA. (d) The left external carotid artery was temporarily released to flush the air and debris through the toe of the orifice. (e) Completed anastomosis of the right common carotid artery and synthetic graft. (f) Completed anastomosis of the left common carotid artery and synthetic graft.
DISCUSSION
Common carotid artery occlusion (CCAO) is a rare disease and its treatment strategies are still not established. However, many bypass treatment options have been reported earlier.[
However, carotid-to-carotid crossover bypass possesses certain risks as well. First, bilateral cervical surgery may cause bilateral laryngeal nerve injury, resulting in dysphagia and airway constriction. Second, insufficiency of blood supply to the affected side or steal phenomenon of the unaffected side may arise. Until the surgery is completed, it remains uncertain whether the unilateral carotid artery can supply the bilateral hemisphere with sufficient blood flow. Our postoperative SPECT showed no remarkable change in cerebral blood flow as per our preoperative expectation; however, the patient recovered well and had no recurrent infarction after bypass surgery. Given that the blood flow of the unilateral CCA is shared with the bilateral ICA, one of the problems of crossover bypass is the uncertainty of blood supply. Third, the normal carotid artery must be clamped during anastomosis, as it may cause ischemic stroke in the unaffected hemisphere. Conversely, the ECA on the unaffected side is an ideal inflow vessel to avoid ischemic complication in the unaffected hemisphere. Unfortunately, in our case, the position of the right carotid bifurcation was so high that we found it difficult to expose the ECA for anastomosis. Therefore, we had to choose the right CCA as an inflow vessel despite the potential risk for ischemic complications.
Recently, endovascular treatment and hybrid procedures have been reported.[
Therefore, we performed a crossover bypass that had a favorable outcome for this patient. Indeed, this approach has risks of complications and uncertainty of blood supply, but it also has some advantages over other treatment options. Hence, carotid-to-carotid crossover bypass can be considered a treatment option for patients with CCA occlusion.
CONCLUSION
Carotid-to-carotid bypass is a durable treatment option for symptomatic CCAO and has advantages over other treatment options. Further studies are needed to better establish its safety and efficacy.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Belczak S, Mulatti GC, Abrão SR, da Silva ES, Aun R, Puech-Leão P. Common carotid artery occlusion: A single-center experience in 40 cases. Int J Angiol. 2016. 25: 39-43
2. Belkin M, Mackey WC, Pessin MS, Caplan LR, O’Donnell TF. Common carotid artery occlusion with patent internal and external carotid arteries: Diagnosis and surgical management. J Vasc Surg. 1993. 17: 1019-27
3. Chen C, Ye Z, Luo L, Guo Y, Chang Y, Ning X. Carotidcarotid artery crossover bypass with a synthetic vascular graft for symptomatic Type 1A common carotid artery occlusion. World Neurosurg. 2018. 111: e286-93
4. Hecht N, Wessels L, Fekonja L, von Weitzel-Mudersbach P, Vajkoczy P. Bypass strategies for common carotidartery occlusion. Acta Neurochir (Wien). 2019. 161: 1993-2002
5. Hiu T, Kitagawa N, Suyama K, Nagata I. Progressing Takayasu arteritis successfully treated by common carotid-internal carotid crossover bypass grafting: Technical case report. Neurosurgery. 2008. 62: E1178-9
6. Hsu JC, Tsai HL. Endovascular recanalization of common carotid artery Total occlusion: Two case reports andliterature review. CVIR Endovasc. 2020. 3: 6
7. Klonaris C, Kouvelos GN, Kafeza M, Koutsoumpelis A, Katsargyris A, Tsigris C. Common carotid artery occlusion treatment: Revealing a gap in the current guidelines. Eur J Vasc Endovascularsurg. 2013. 46: 291-8
8. Law MM, Colburn MD, Moore WS, Quiñones-Baldrich WJ, Machleder HI, Gelabert HA. Carotid-subclavian bypass for brachiocephalic occlusive disease, Choice of conduit and long-term follow-up. Stroke. 1995. 26: 1565-71
9. Li ZY, Chen C, Ling C, He HY, Luo L, Li H. Surgical procedures including carotid-carotid crossover bypass and ring-stripping hybrid operation for Rile’s Type 1A common carotid artery occlusion: An experience of 6cases. Ann Transl Med. 2020. 8: 439
10. Martin RS, Edwards WH, Mulherin JL, Edwards WH. Surgical treatment of common carotid arteryocclusion. Am J Surg. 1993. 165: 302-6
11. Matsuda Y, Koyama T. Evaluation of revascularization after total arch replacement in common carotid arteryocclusion. World J Clin Cases. 2018. 6: 6-10
12. Morofuji Y, Matsunaga Y, Izumo T. Carotid-carotid bypass for a carotid artery aneurysm. World Neurosurg. 2020. 138: 7-8
13. Ozsvath KJ, Roddy SP, Darling RC, Byrne J, Kreienberg PB, Choi D. Carotid-carotid crossoverbypass: Is it a durable procedure?. J Vasc Surg. 2003. 37: 582-5
14. Powers WJ, Clarke WR, Grubb RL, Videen TO, Adams HP, Derdeyn CP. Extracranial-intracranial bypass surgery for stroke prevention in hemodynamic cerebral ischemia: The carotid occlusion surgery study randomized trial. JAMA. 2011. 306: 1983-92
15. Riles TS, Imparato AM, Posner MP, Eikelboom BC. Common carotid occlusion. Assessment of the distalvessels. Ann Surg. 1984. 199: 363-6
16. Sullivan TM. Subclavian-carotid bypass to an isolated carotid bifurcation: A retrospective analysis. Ann Vasc Surg. 1996. 10: 283-9