- Department of Neurosurgery, Seoul National University Hospital, Seoul, Korea.
- Department of Neurosurgery, Seoul National University Children’s Hospital, Seoul, Korea.
Correspondence Address:
Sung Ho Lee, Department of Neurosurgery, Seoul National University Hospital, Seoul, Korea.
DOI:10.25259/SNI_149_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sejin Choi1, Sung Ho Lee1, Kyunghyun Kim2, Kang Min Kim1, Won-Sang Cho1, Hyun-Seung Kang1, Jeong Eun Kim1. A child who presented with cerebral infarction: Clipping combined with bypass surgery of a thrombosed giant aneurysm. 31-Mar-2023;14:115
How to cite this URL: Sejin Choi1, Sung Ho Lee1, Kyunghyun Kim2, Kang Min Kim1, Won-Sang Cho1, Hyun-Seung Kang1, Jeong Eun Kim1. A child who presented with cerebral infarction: Clipping combined with bypass surgery of a thrombosed giant aneurysm. 31-Mar-2023;14:115. Available from: https://surgicalneurologyint.com/surgicalint-articles/12227/
Abstract
Background: Cerebral aneurysms are not common among children and most of them are presented with subarachnoid hemorrhage or mass effect. Here, we describe a rare case of a pediatric giant aneurysm presented with cerebral infarction.
Case Description: A 38-month-old boy visited the emergency room due to left hemiparesis and left central type facial palsy. Initial magnetic resonance imaging showed acute cerebral infarction on the right basal ganglia and coronal radiata. Furthermore, a thrombosed aneurysm with a diameter of 30.57 mm at the frontal branch of the right middle cerebral artery was observed. A right pterional craniotomy with Sylvian dissection was performed. Superior and inferior divisions of the frontal branch originating from the aneurysm were identified. The superior division was cutoff from an aneurysm and clipping saving the inferior division was done. Subsequently, end-to-end anastomosis was done between a parietal branch of the superficial temporal artery and a superior division from the aneurysm. No acute complication from the operation was observed. Motor power of the left upper extremity recovered after rehabilitation, while fine motor impairment remained 6 months after the surgery.
Conclusion: This case illustrates successful treatment of a pediatric giant aneurysm with extremely rare presentation of cerebral infarction, under a meticulous surgical plan and ad hoc modification.
Keywords: Bypass surgery, Clipping, Pediatric cerebral infarction, Pediatric giant aneurysm
INTRODUCTION
Pediatric cerebral aneurysms are not common and it comprises about 0.5–4.6% of all aneurysms.[
CASE DESCRIPTION
A 38-month-old boy, 96 cm in height and 14 kg in weight, visited the emergency room due to gait disturbance and dysarthria started abruptly 1 day before the visit. Left hemiparesis of motor grade III and left central type facial palsy were observed. Initial brain computed tomography (CT) and magnetic resonance imaging (MRI) showed acute cerebral infarction on the right basal ganglia and coronal radiata [
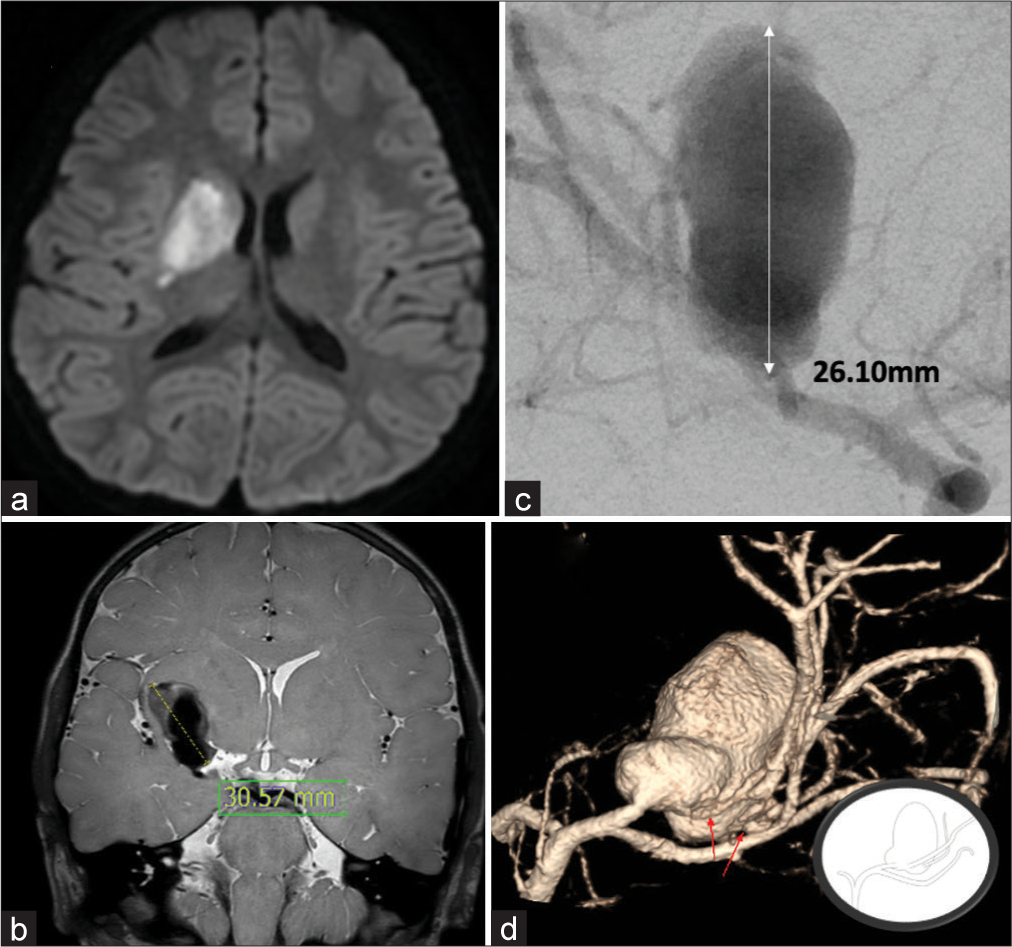
Figure 1:
Preoperative images. (a) MR diffusion weighted images at presentation demonstrated cerebral infarction at right basal ganglia and corona radiata. (b) T2 weighted image-vessel wall MR showed aneurysm with partial thrombosis (30.57 mm) and enhancement of superior aspect. (c) Digital subtracted angiography confirmed a large saccular aneurysm (14.9 × 26.1 mm) at the right middle cerebral artery. (d) After early bifurcation of middle cerebral artery, the aneurysm was originated from the frontal branch. Superior and inferior divisions of frontal branch are branching out from the aneurysm dome. (arrows) (inlet).
The patient was delivered at full term, previously healthy, and reached developmental milestones. Echocardiography, blood culture, vasculitis workup, and gene panel were done and these showed no sign of mycotic aneurysm or underlying disease such as coarctation of the aorta, polycystic kidney disease, fibromuscular dysplasia, tuberous sclerosis, Ehlers-Danlos syndrome, and Marfan syndrome. Daily Aspirin of 50 mg (3 mg/kg) was given, and after 1 month, follow-up MRI showed decrease of thrombus in the aneurysm, but size of aneurysm was not changed. Several surgical options were consided based on preoperative images, to obliterate aneurysm and maintain distal flow simultaneously [
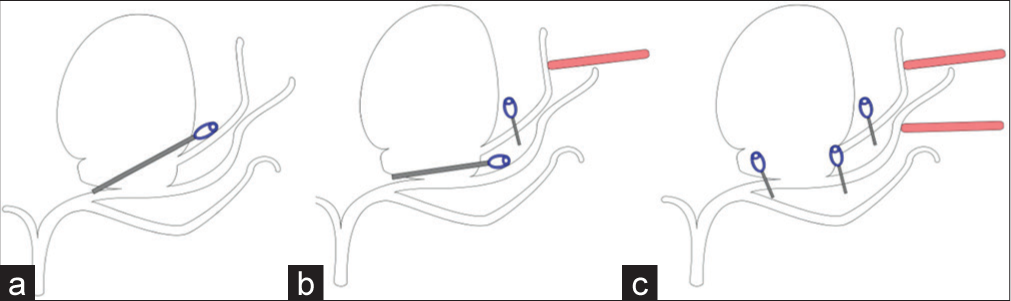
Figure 2:
Illustration of operation plan. Gray blade with blue spring represent aneurysm clips. Possible surgical options based on the preoperative images are as follows: (a) direct clipping if possible. (b) Neck clipping combined with anastomosis of superior division of the frontal branch-graft from superficial temporal artery (red bar). (c) Trapping of aneurysm combined with double-barrel bypass via two branches of superficial temporal arteries (red bars).
After curvilinear skin incision, subgaleal dissection was done to acquire the scalp flap containing the right superior temporal artery (STA). A distal end of STA’s parietal branch was cut and prepared for anastomosis. A wide and right pterional craniotomy with Sylvian dissection was performed. Proximal to the aneurysm, we noted prebifurcation of M1. Branches originating from the aneurysm were identified – superior devision of the frontal branch, inferior division of the frontal branch, and lenticulostriate arteries from the frontal branch [
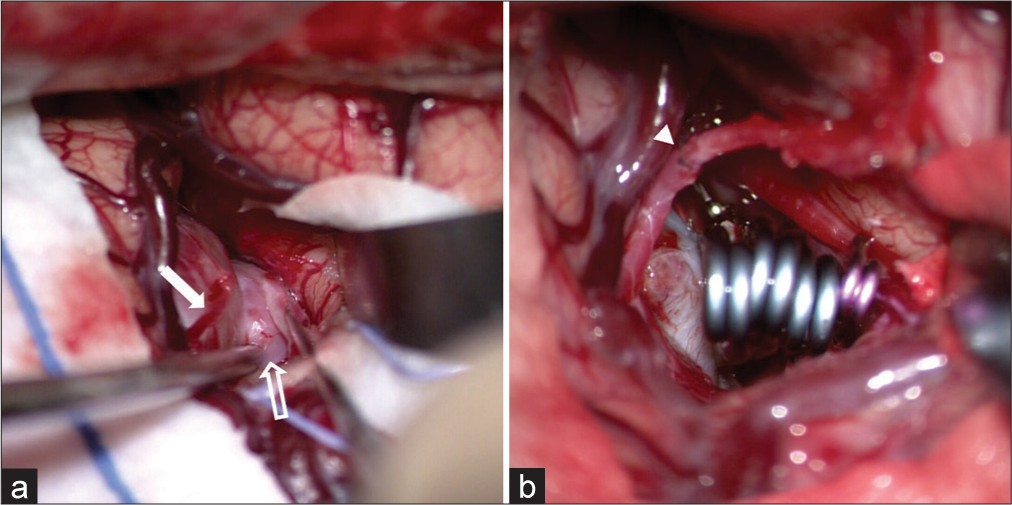
Figure 3:
Operative pictures. (a) the aneurysm was originated from the frontal branch of the middle cerebral artery. Superior (solid arrow) and inferior (open arrow) divisions of frontal branch are branching out from the aneurysm. (b) Clipping of main sac was done using multiple fenestrated straight clips. And then the superior division of frontal branch and superior temporal artery’s parietal branch were anastomosed in end-to-end fashion. (arrowheads).
Initially, we attempted direct clipping avoiding the superior divison of the frontal branch, but it made the parent artery compromised. Thus, the superior division was cut from an aneurysm and clipping was done for the main sac with one 12 mm fenestrated straight clip. Two 9 mm straight fenestrated clips were used as booster. Inferior division of the frontal branch was preserved [
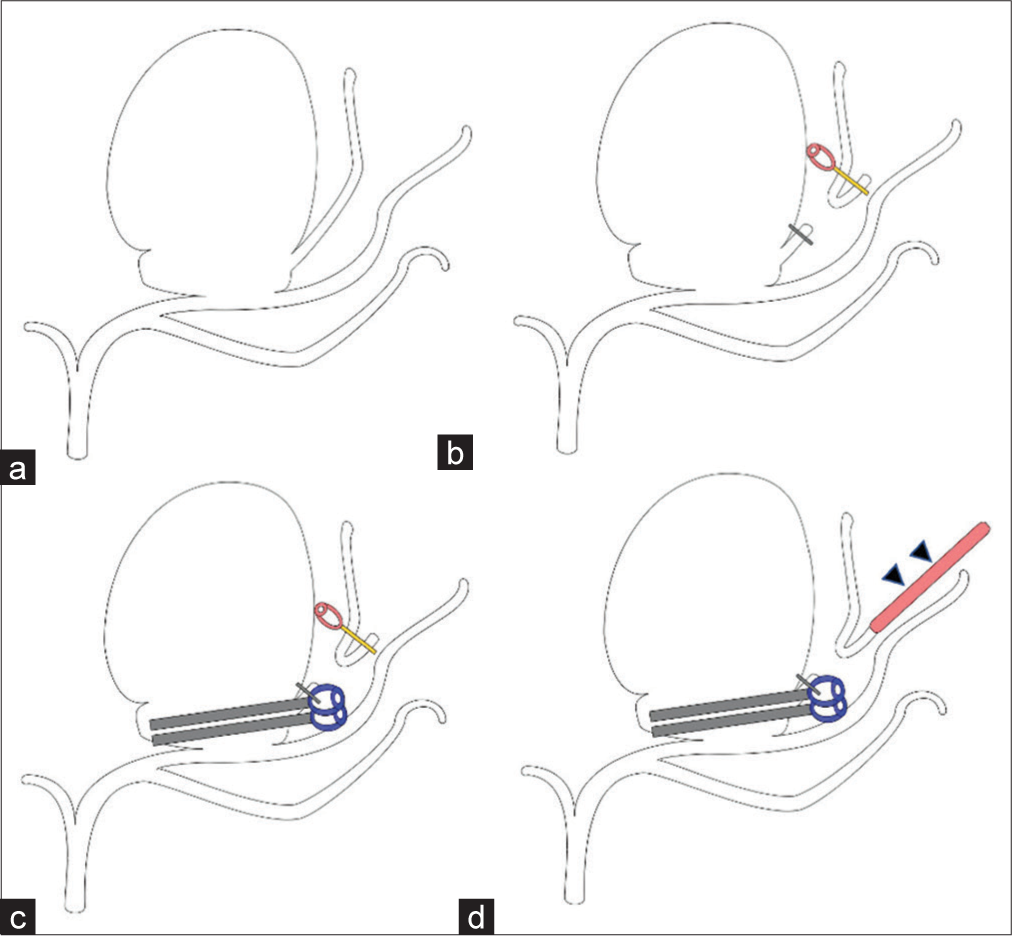
Figure 4:
Illustration of operation. Gray blade with blue spring represents fenestrated straight clips while gold blade with violet spring means temporary mini clips. (a) After early bifurcation of middle cerebral artery, the aneurysm was originated from the frontal branch. Superior and inferior divisions of frontal branch are branching out from the aneurysm. (b) Superior division of frontal branch was cut to enable clipping without compromising parent artery. (c) Clipping of main sac was done using multiple fenestrated straight clips. (d) Superior division of frontal branch and distal end of superior temporal artery’s parietal branch (arrowheads, red bar) were anastomosed in end-to-end fashion.
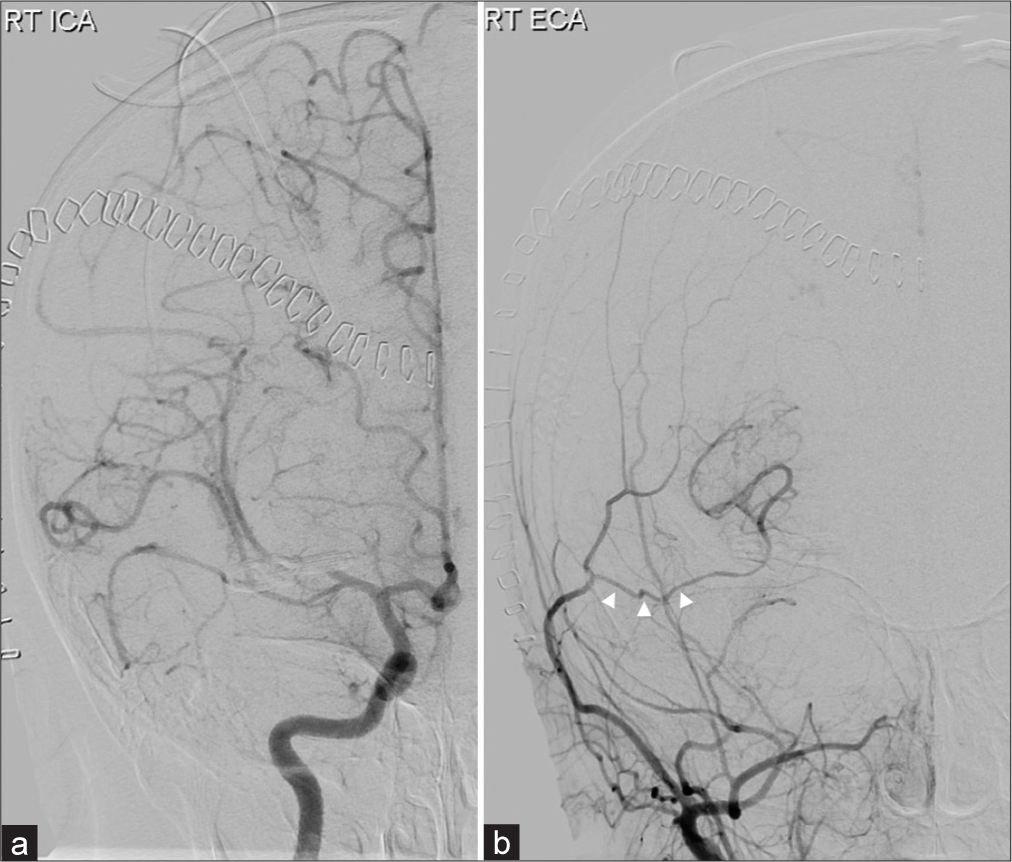
No acute complication from the operation was observed and the patient had no new-onset neurologic deficit. Digital subtracted angiography at postoperative day 3 showed no residual aneurysmal sac and good flow was observed from the donor – right STA parietal branch – to the recipient – M2 area [
DISCUSSION
In this report, we illustrated a rare case of giant pediatric aneurysm that presented as cerebral infarction. One study that reviewed papers on pediatric “giant” aneurysm published from 1990 to 2012 showed that there was no case with cerebral infarction (even though partial thrombosis was present in 23.9% of cases).[
Revascularization techniques involving bypass surgery are one of treatment options for pediatric giant aneurysms, while clipping is practiced more widely according to the previous reports.[
In this case, different surgical approaches were considered preoperatively to achieve the goals of complete ligation of aneurysm and preservation of distal blood flow. Since pediatric aneurysms recur more often than adults counterpart, to minimize the chance of recurrence, complete obliteration was to be achieved. Clipping without bypass was one of the best options [
Endovascular treatment was not chosen in this case for a couple of reasons even though the previous studies showed no difference in long-term outcome between endovascular and surgical treatments for ruptured or unruptured pediatric intracranial aneurysms.[
Sylvian fissure of children is not fully developed and it makes wide dissection difficult. Furthermore, small-vessel size makes bypass surgery challenging. The patient of this case was 38-month-old whose head circumference was 70% of adult head circumference but vessel diameters of recipient candidates were very small (about 1 mm). Moreover, even though the aneurysm was located at the frontal branch of MCA after the bifurcation, as it was bifurcated early, small perforators like lateral lenticulostriate arteries were present. A small perforator was sacrified inevitably, but it did not cause additional neurologic deficit.
CONCLUSION
We illustrated the successfully treated case of a giant MCA aneurysm of a very young age, with an extremely rare presentation – a cerebral infarction. We learned from this case that it is important to make meticulous surgical plan in advance and to modify surgical methods ad hoc according to intraoperative situations. This microsurgical solution of bypass surgery combined with clipping might be helpful to other challenging cases of pediatric giant aneurysms.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflict of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Alamanda VK, Tomycz L, Velez D, Singer RJ. Direct, high-flow bypass for a pediatric giant, fusiform aneurysm of the inferior division of M2: Case report and review of literature. J Surg Tech Case Rep. 2012. 4: 53-7
2. Baeesa SS, Bakhaidar M, Almekhlafi MA, Madani TA. Human immunodeficiency virus-associated cerebral aneurysmal vasculopathy: A systematic review. World Neurosurgery. 2016. 87: 220-9
3. Jian BJ, Hetts SW, Lawton MT, Gupta N. Pediatric intracranial aneurysms. Neurosurg Clin N Am. 2010. 21: 491-501
4. Kalani MY, Elhadi AM, Ramey W, Nakaji P, Albuquerque FC, McDougall CG. Revascularization and pediatric aneurysm surgery. J Neurosurg Pediatr. 2014. 13: 641-6
5. Mura J, Torche E, Riquelme F, Parra M, Julio R. Three-year-old patient with giant MCA aneurysm treated by trapping-resection plus STA-MCA bypass. Case report. Childs Nerv Syst. 2012. 28: 169-73
6. Nam SM, Jang D, Wang KC, Kim SK, Phi JH, Lee JY. Characteristics and treatment outcome of intracranial aneurysms in children and adolescents. J Korean Neurosurg Soc. 2019. 62: 551-60
7. Parkinson RJ, Eddleman CS, Batjer HH, Bendok BR. Giant intracranial aneurysms: Endovascular challenges. Neurosurgery. 2006. 59: S103-12 discussion S3-13
8. Pruvot AS, Curey S, Derrey S, Castel H, Proust F. Giant intracranial aneurysms in the paediatric population: Suggested management and a review of the literature. Neurochirurgie. 2016. 62: 20-4
9. Schaller B. Extracranial-intracranial bypass to reduce the risk of ischemic stroke in intracranial aneurysms of the anterior cerebral circulation: A systematic review. J Stroke Cerebrovasc Dis. 2008. 17: 287-98
10. Strickland BA, Attenello F, Russin JJ. Extracranial to intracranial bypass for the treatment of cerebral aneurysms in the pediatric population. J Clin Neurosci. 2016. 34: 6-10
11. Yasin JT, Wallace AN, Madaelil TP, Osbun JW, Moran CJ, Cross DT. Treatment of pediatric intracranial aneurysms: Case series and meta-analysis. J Neurointerv Surg. 2019. 11: 257-64