- Brain Tumor and Neuro-Oncology Center, Cleveland Clinic, Cleveland, Ohio, United States.
- Department of Neurological Surgery Cleveland Clinic, Cleveland, Ohio, United States.
- Department of Anesthesiology, Cleveland Clinic, Cleveland, Ohio, United States.
Correspondence Address:
Pablo F. Recinos
Brain Tumor and Neuro-Oncology Center, Cleveland Clinic, Cleveland, Ohio, United States.
Department of Neurological Surgery Cleveland Clinic, Cleveland, Ohio, United States.
DOI:10.25259/SNI_737_2020
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Miguel A. Recinos1, Jason Hsieh2, Hussain Mithaiwala3, Joti Juneja Mucci3, Pablo F. Recinos1,2. A rare appearance of the trigeminocardiac reflex during resection of posterior parasagittal meningioma. 26-Apr-2021;12:183
How to cite this URL: Miguel A. Recinos1, Jason Hsieh2, Hussain Mithaiwala3, Joti Juneja Mucci3, Pablo F. Recinos1,2. A rare appearance of the trigeminocardiac reflex during resection of posterior parasagittal meningioma. 26-Apr-2021;12:183. Available from: https://surgicalneurologyint.com/surgicalint-articles/10762/
Abstract
Background: Although a well-recognized phenomenon of the tentorium and posterior fossa, the trigeminocardiac reflex (TCR) has been rarely reported during surgery involving the posterior falx cerebri.
Case Description: We present the case of a 63-year-old woman who underwent repeat resection of an atypical parasagittal meningioma involving the posterior falx. During resection, TCR was repeatedly elicited during manipulation and coagulation of the falx. Air embolism and cardiac etiologies were initially considered while TCR was not suspected, given the location. Ultimately, TCR was recognized when asystole self-resolved upon cessation of stimulus and due to its reproducibility.
Conclusion: Awareness by the anesthesiologist and neurosurgeon of the possibility of TCR during falcine procedures can help with rapid identification to avoid a potentially catastrophic outcome.
Keywords: Air embolism, Falx, Nervous tentorii, Trigeminal nerve, Vagus nerve
INTRODUCTION
Elicitation of the trigeminocardiac reflex (TCR) by mechanical, electrical, or chemical stimuli may result in the sudden onset of bradycardia, hypotension, and gastric hypermotility.[
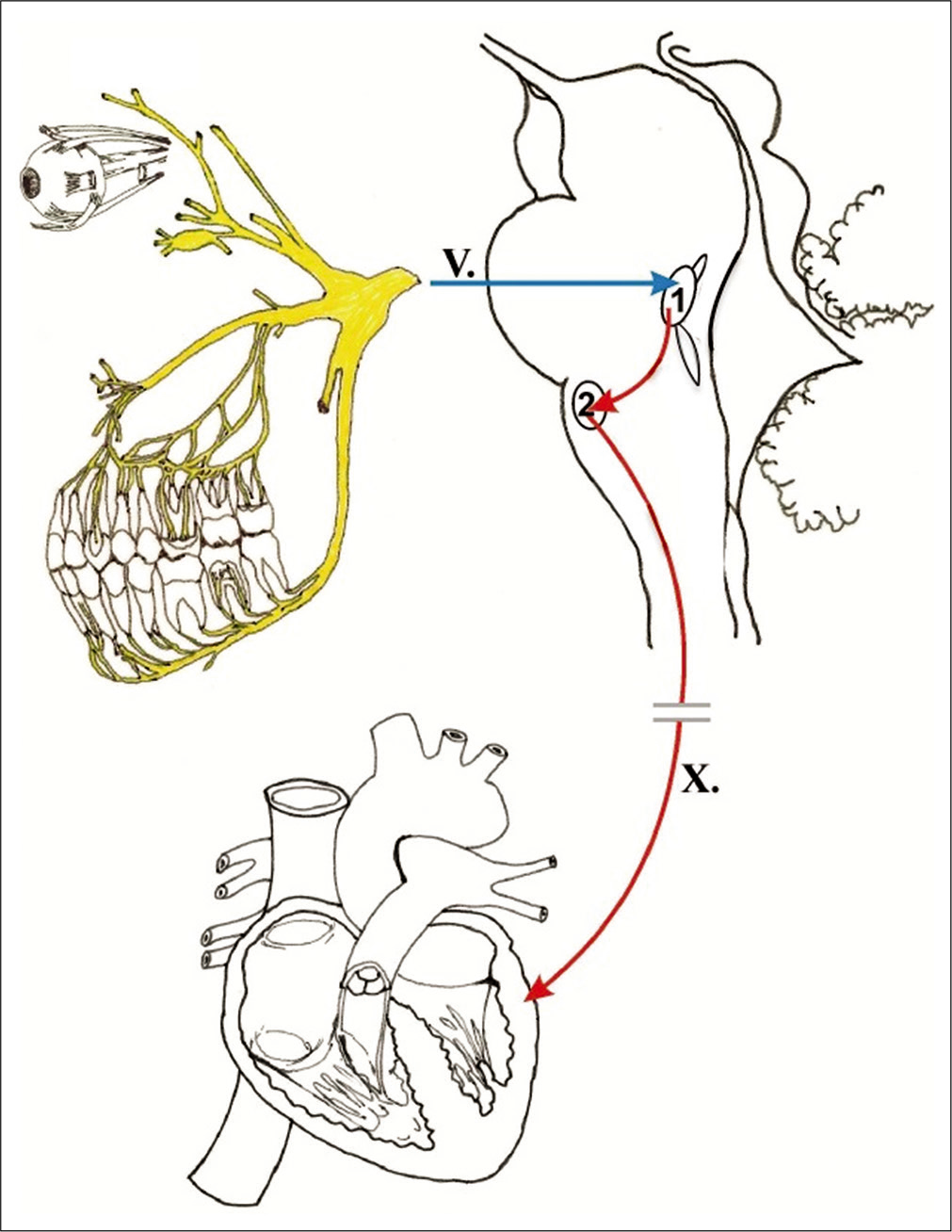
Figure 1:
Afferent stimulus that may elicit the trigeminocardiac reflex travel along any of the main branches of CN V. The 1st division (V1) innervates the falx through a recurrent branch, the nervous tentorii. Stimuli (blue arrow) travel through the Gasserian ganglion to the chief sensory nucleus of the trigeminal nerve (1). The spinal nucleus of the trigeminal nerve is also involved in processing nociceptive stimuli. Interneural fibers (upper red arrow) join the trigeminal nucleus and the dorsal motor nucleus of the vagus nerve (2), from which efferent vagal parasympathetic preganglionic fibers (lower red arrow) emerge to travel to diverse systemic locations including parasympathetic ganglia responsible for modulation of the heart rate through the sinoatrial node. This figure is used with permission from the publishers. Reference: Abdulazim A, Martin N, Sadr-Eshkevari P, Prochnow N, Sandu N, Bohluli B, et al. Trigeminocardiac reflex in neurosurgery - current knowledge and prospects. In: Signorelli F, editor. Explicative Cases of Controversial Issues in Neurosurgery. London: InTech; 2012. p. 3-18.
Surgeons operating on parasagittal meningiomas and other lesions in the region of the cerebral venous sinuses must remain vigilant for the possibility of venous air embolism (AE). Inadvertent entry into venous sinuses can allow the entry of air into the low-pressure venous return, resulting in entrapped air in the right atrium. Potential consequences of AE include hypotension, myocardial infarction, and arrythmia. If air is instead shunted to the systemic circulation through a patent foramen ovale, sequelae may include arterial ischemia including cerebral infarction.[
We present a unique case of TCR, that occurred during resection of an atypical parasagittal meningioma found in the middle to posterior third of the superior sagittal sinus (SSS). While preparation for AE was performed and there was a heighted awareness of the possibility of cardiac events due to the patient’s cardiac risk factors, elicitation of TCR was unexpected and knowledge of its possibility in future similar cases would help to more rapidly recognize TCR and guide intraoperative management.
CASE REPORT
The patient was a 63-year-old woman with a recurrent parafalcine meningioma. She had presented originally 6 years prior with focal right lower extremity motor seizures and was found to have an approximately 4 cm bilateral parafalcine meningioma at the paracentral lobule that was eccentric to the left. Her initial resection was aborted due to sustained ST elevations before dural opening. After cardiac catheterization, evaluation, and clearance, she underwent resection 1 month later through a bicoronal approach. The tumor was heavily calcified, invading, and densely adherent to the falx and sinus, and although sinus bleeding was encountered, a Simpson Grade III resection was achieved. The sinus was preserved. Pathology revealed the WHO Grade II atypical meningioma. Notably, she was later diagnosed with coronary artery disease.
She underwent adjuvant radiotherapy (54 Gy) and later twice underwent gamma knife radiosurgery for progressive disease at 4 years and 5 years postoperatively. Six years postoperatively, she experienced worsening seizures and imaging revealed meningioma recurrence. Preoperative vascular imaging showed SSS occlusion [
Perioperatively, a central line was placed, and precordial Doppler was used due to concern for possible AE. We opened and extended her prior bicoronal incision. After significant dissection through scar tissue and extension of craniotomy, we began resection of the tumor. During tumor resection, the falx was being coagulated and the patient became profoundly bradycardic, then asystolic [
After discussion between the anesthesia and neurosurgery teams, AE and a cardiac etiology were ruled out and surgery resumed. By careful exploration of the tumor-falcine-sinus interface, we confirmed that the sinus was occluded by tumor and began to coagulate it again to sacrifice it to improve resection. Given the likely chronic nature of her sinus occlusion, we felt confident that this would not result in venous congestion. During repeat coagulation of the SSS and posterior falx, profound bradycardia occurred again and self-resolved upon cessation of coagulation. This was repeatedly reproducible. After sacrifice of the sinus and falx in the region of the tumor and careful dissection, we were able to achieve a Simpson Grade I resection. The remainder of the case was uncomplicated.
DISCUSSION
The TCR was first described in rabbits by Kumada et al. in 1977 during neurostimulation experiments and first reported in humans by Brown and Preul in 1988 as a trigeminal depressor response.[
The falx is innervated by the nervus tentorii, which is a recurrent branch of cranial nerve V1.[
TCR can usually be terminated by simple removal of the triggering stimulus and intraoperatively may be coupled with anesthetic maneuvers to correct mild hemodynamic and metabolic perturbations. If hypotension and bradycardia do not correct immediately, anticholinergic agents (such as glycopyrrolate or atropine) can be administered to mitigate these factors. In extreme cases where stimulus removal and administration of anticholinergic fails to terminate TCR, cardiac life support should be utilized to avoid potentially fatal hemodynamic instability.[
Although TCR was the physiological explanation in this case, monitoring for AE is important when performing high-risk meningioma operations such as the one described in this report. The three most common methods used to detect AE are a right atrial central venous pressure line (that can also aspirate air), transesophageal echocardiography, and precordial Doppler.[
In the present case, after the first incident of bradycardia was reported, AE was immediately considered, as was a cardiac event, due to the patient’s history of cardiac disease. However, in cases involving the falcine region, TCR should also be taken into consideration to prevent asystole. The important distinction is that asystole from TCR was the only potential etiology that could self-resolve by immediately stopping the initiating stimulus, whereas asystole from AE or a cardiac etiology would likely necessitate cardiopulmonary resuscitation. Both the anesthesia and neurosurgical teams should be aware that TCR can result from stimulation of the posterior falx. By rapidly communicating the changes seen, the offending stimulus can be immediately stopped before the patient progressing to asystole and potential cardiac arrest. Typically, the anesthesia team goes through an algorithm when significant cardiovascular changes occur. The first step is typically to tell neurosurgeon to stop any stimulus to assess for resolution of cardiac changes. This case further highlights the importance of this step in such an algorithm.
CONCLUSION
Awareness by the anesthesiologist and neurosurgeon of the possibility of TCR during falcine procedures can help with rapid identification to avoid a potentially catastrophic outcome.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Recinos is a consultant for Stryker.
References
1. Abdulazim A, Stienen MN, Sadr-Eshkevari P, Prochnow N, Sandu N, Bohluli B, Signorelli F.editors. Trigeminocardiac reflex in neurosurgery current knowledge and prospects. Explicative Cases of Controversial Issues in Neurosurgery. London: InTech; 2012. p. 3-18
2. Bauer DF, Youkilis A, Schenck C, Turner CR, Thompson BG. The falcine trigeminocardiac reflex: Case report and review of the literature. Surg Neurol. 2005. 63: 143-8
3. Borghei-Razavi H, Das P, Maurtua M, Recinos PF. Unusual appearance of trigemino-cardiac reflex during cerebellopontine angle surgery. World Neurosurg. 2018. 112: 298-9
4. Brown JA, Preul MC. Trigeminal depressor response during percutaneous microcompression of the trigeminal ganglion for trigeminal neuralgia. Neurosurgery. 1988. 23: 745-8
5. Das KK, Gosal JS, Sharma P, Mehrotra A, Bhaisora K, Sardhara J. Falcine meningiomas: analysis of the impact of radiologic tumor extensions and proposal of a modified preoperative radiologic classification scheme. World Neurosurg. 2017. 104: 248-58
6. Fayon M, Gauthier M, Blanc VF, Ahronheim GA, Michaud J. Intraoperative cardiac arrest due to the oculocardiac reflex and subsequent death in a child with occult epstein-barr virus myocarditis. Anesthesiology. 1995. 83: 622-4
7. Kumada M, Dampney RAL, Reis DJ. The trigeminal depressor response: A novel vasodepressor response originating from the trigeminal system. Brain Res. 1977. 119: 305-26
8. Raza SM, Gallia GL, Brem H, Weingart JD, Long DM, Olivi A. Perioperative and long-term outcomes from the management of parasagittal meningiomas invading the superior sagittal sinus. Neurosurgery. 2010. 67: 885-93
9. Sahoo BK.editors. Incidence and Complications of Venous Air Embolism in Patients Undergoing Selective Intracranial Surgeries by Using Transesophageal Echocardiography. Thiruvananthapuram, Kerala, India: D.M. Dissertation, Department of Anaesthesiology, Sree Chitra Tirunal Institute for Medical Sciences and Technology; 2017. p.
10. Schaller B, Cornelius JF, Prabhakar H, Koerbel A, Gnanalingham K, Sandu N. The trigemino-cardiac reflex: An update of the current knowledge. J Neurosurg Anesthesiol. 2009. 21: 187-95
11. Schaller B, Probst R, Strebel S, Gratzl O. Trigeminocardiac reflex during surgery in the cerebellopontine angle. J Neurosurg. 1999. 90: 215-20
12. Shaikh N, Ummunisa F. Acute management of vascular air embolism. J Emerg Trauma Shock. 2009. 2: 180
13. Takano K, Kinoshita M, Hashimoto N, Tanigami H, Yoshimine T. A reminder about the trigeminocardiac reflex in surgeries at the posterior third of the falx cerebri. Interdiscip Neurosurg Adv Tech Case Manag. 2014. 1: 47-9