- Department of Clinical Medicine, Executive Health and Concierge Medicine,
- Department of Neurological Surgery, University of Miami Miller School of Medicine, Miami, Florida, United States.
- Department of Neurological Surgery, University of Miami Miller School of Medicine, Miami, Florida, United States.
Correspondence Address:
Adam Shapira Levy, University of Miami Miller School of Medicine, Miami, Florida, United States.
DOI:10.25259/SNI_730_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Stephen V. Avallone1, Adam S. Levy2, Robert M. Starke3. A rare case of Streptococcus anginosus infectious intracranial aneurysm: Proper management of a poor prognosis. 30-Sep-2021;12:487
How to cite this URL: Stephen V. Avallone1, Adam S. Levy2, Robert M. Starke3. A rare case of Streptococcus anginosus infectious intracranial aneurysm: Proper management of a poor prognosis. 30-Sep-2021;12:487. Available from: https://surgicalneurologyint.com/surgicalint-articles/11140/
Abstract
Background: Infectious intracranial aneurysms (IIAs), sometimes referred to as cerebral mycotic aneurysms, are an uncommon but feared compilation of bacterial endocarditis, occurring in up to 5% of all bacterial endocarditis cases. While IIAs carry a low risk of rupture, a ruptured mycotic aneurysm carries devastating neurologic consequences with up to an 80% mortality rate secondary to subarachnoid and intracerebral hemorrhage.
Case Description: A 69-year-old man undergoing antibacterial therapy for Streptococcus anginosus endocarditis with aortic insufficiency and root abscess presented to the ED with multiple seizures and left-sided weakness. MRI of the head revealed right frontal and temporal abscesses with evidence of scattered septic emboli and subarachnoid hemorrhage. CTA of the head revealed a ruptured 1 mm distal middle cerebral artery mycotic aneurysm. Prior to undergoing surgery, the patient began to decline, becoming lethargic, and failing to respond to commands. The patient underwent endovascular Onyx embolization. After the procedure, the patient remained with partial status epilepticus and was discharged to rehabilitation. Over the following months, the patient made a great recovery and was able to undergo aortic and mitral valve replacement 5 months after neurosurgical intervention.
Conclusion: This favorable outcome is the result of a tremendous deal of long-term coordination and efficient communication between neurosurgery, cardiology, neurology, physical medicine and rehabilitation, and primary care.
Keywords: Endovascular, Infectious intracranial aneurysm, Mycotic aneurysm, Subarachnoid hemorrhage
INTRODUCTION
As with most major cerebrovascular events, rupture of an infectious intracranial aneurysm (IIA) carries a relatively poor prognosis with a high morbidity and mortality rate.[
IIAs are an uncommon but feared phenomena most commonly associated with bacterial endocarditis with an incidence of up to 5% in all bacterial endocarditis cases.[
Here, we describe a case of bacterial endocarditis complicated by an IIA of rare etiology with a good outcome that required extensive interdisciplinary long-term care and follow-up. The case required months of medical management, two separate surgical interventions, and hours of physical rehabilitation to obtain a favorable outcome. This case highlights the importance of effective interdepartmental communication in achieving optimal care.
CASE REPORT
Presentation
A 69-year-old male with known hypertension and poor dentition presented to the hospital with 5 months of dry cough, night sweats, and weight loss. Physical exam revealed a new 2/6 diastolic murmur. Investigative TEE showed a left aortic perivalvular abscess with vegetations and severe aortic insufficiency. The diagnosis of native valve subacute endocarditis was made, with blood cultures positive for Streptococcus anginosus. The patient was discharged on a 1-month course of IV Ceftriaxone and scheduled for a minimally invasive aortic valve replacement with possible aortic root reconstruction on completion of antibiotic therapy. On day 26/28 of antibiotic therapy, the patient presented to his local emergency room after having experienced a first-time seizure and loss of consciousness. He had no personal history or known family history of seizures or seizure disorders.
On admission, physical exam showed elevated blood pressure at 151/61 with a regular heart rate of 68, a faint diastolic murmur at the base, and obvious favoring of the right upper and lower extremities without pronator drift. Physical exam was otherwise unremarkable. The patient was given Levetiracetam 1000 mg and Dexamethasone 6 mg for seizure management.
Investigations
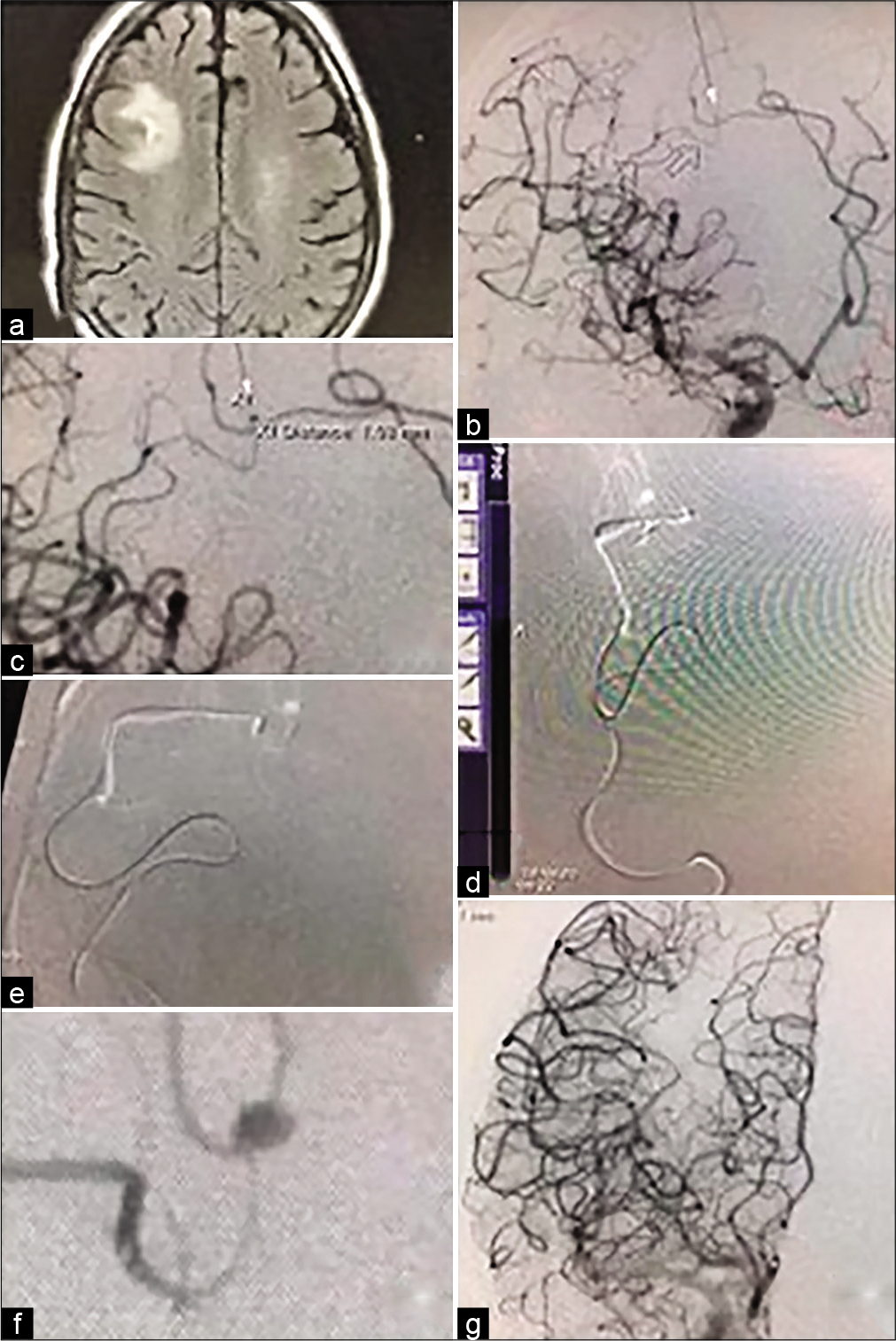
At the patient’s local ED, initial contrast CT of the head showed a 1.5 cm rim enhancing lesion with no reported hydrocephalus. He was transferred to our center for further care. On the morning following transfer, the patient suffered a second left-sided seizure at which point an MRI with and without contrast was performed. The MRI revealed a 1.4 × 1.4 × 1.6 cm rim-enhancing lesion in the right middle frontal gyrus with surrounding vasogenic edema suggestive of a pyogenic abscess [
Figure 1:
(a) MRI Flair sequence shows right frontal lesion with surrounding edema and subarachnoid hemorrhage consistent with cerebral abscess and ruptured mycotic aneurysm. (b and c) Digital subtraction angiography anterior-posterior and oblique views shows mycotic aneurysm measuring 1 mm. (d and e) show roadmap angiography with microcatheter approach to the aneurysm. (f) Microcatheter injection just proximal to the mycotic aneurysm. (g) Internal carotid artery digital subtraction angiography after onyx embolization of the mycotic aneurysm with no residual filling and no unintended thromboembolic phenomena.
Treatment
After visualization of the suspected abscess on MRI [
Vascular access was first gained through the right femoral artery. An Envoy guide catheter was advanced to the right internal carotid artery through roadmap assistance at which point a 3D angiogram was taken to better visualize the right MCA. Due to the tortuosity of the patient’s right iliac system, the guide catheter was unable to be advanced any further. Instead, left femoral artery access was gained and the vascular catheter was advanced in a similar fashion. The guide catheter was advanced to the M2 branch of the right MCA where it was exchanged for an intermediate catheter over a microcatheter over a Synchro microwire. A superselective M3 and M4 angiogram were then performed, allowing for visualization of the 1 mm infectious aneurysm in the cortical M4 frontal MCA branch [
One day following surgery, the patient developed seizures with partial status epilepticus. CT of the brain showed a hyperdensity in the subarachnoid space with subtle ventriculomegaly due to rupture of the IIA with subarachnoid hemorrhage (Modified Fisher Scale grade 2/Hunt and Hess grade 3) status post embolization. The patient was closely monitored for deterioration of symptoms. Two days later, follow-up CT of the brain revealed stable subarachnoid and intraventricular hemorrhage with no hydrocephalus. It was ultimately decided no external ventricular drain would be placed given the lack of hydrocephalus and stability of bleed. The patient remained in the NICU for 22 days where he was placed on Levetiracetam, Clobazam, and Lacosamide for seizure control. During this time, the patient was followed closely by neurology with daily electroencephalograms and by pulmonary/critical care as he remained intubated on mechanical ventilation post procedure. Infectious disease also followed the patient for infective endocarditis and later began triple antibiotic treatment with Vancomycin, Cefepime, and Metronidazole for ventilator associated pneumonia and right middle frontal gyrus abscess.
On leaving the NICU, the patient was evaluated by physical medicine and rehabilitation who initiated aggressive physical therapy, occupational therapy, and speech therapy. The patient was discharged to a hospital-adjacent rehabilitation center 1 week later where he continued to undergo physical, occupational, and speech therapy for 1 month.
Four months after the patient’s initial hospital discharge, he was deemed a suitable candidate to undergo cardiothoracic surgery. The patient underwent an aortic and mitral valve replacement without complication and was discharged home after 4 days of hospital care.
Outcome and follow-up
For 6 months, the patient remained out of the hospital with mild lifestyle modifications and changes to mood (including some confusion) which improved on cessation of steroid therapy.
The patient was readmitted to the hospital 6 months after cardiac surgery when he suffered a left sided seizure with associated Todd’s paralysis attributed to possible nonadherence. During the admission, he suffered a pulmonary embolism for which an IVC filter was placed. He was discharged home again with daily speech, occupational, and physical therapy. Since the episode, the patient had no major hospital admission and has been adherent to his antiseizure regimen of Levetiracetam, Lacosamide, Topiramate, and Clobazam.
At present, the patient is 1½ years status post initial rupture of the IIA. The patient is currently alive, and his mood has significantly improved per both his family and healthcare team. He has suffered several minor seizures in the interim due to medication titrations. However, he has reached the point where he is again confident enough and possibly eligible to begin driving school.
DISCUSSION
IIAs, sometimes referred to as cerebral mycotic aneurysms, are an uncommon but feared compilation of bacterial endocarditis. While bacterial endocarditis is the underlying cause of IIAs in nearly all modern cases, valve surgery is typically postponed until resolution of the aneurysm due to use of perioperative anticoagulation. IIAs have been found to occur in up to 5% of bacterial endocarditis cases, and typically present with multiple lesions.[
While the aforementioned algorithms exist, it is important to note that no randomized control trials exist to compare management of IIAs due to their low prevalence.[
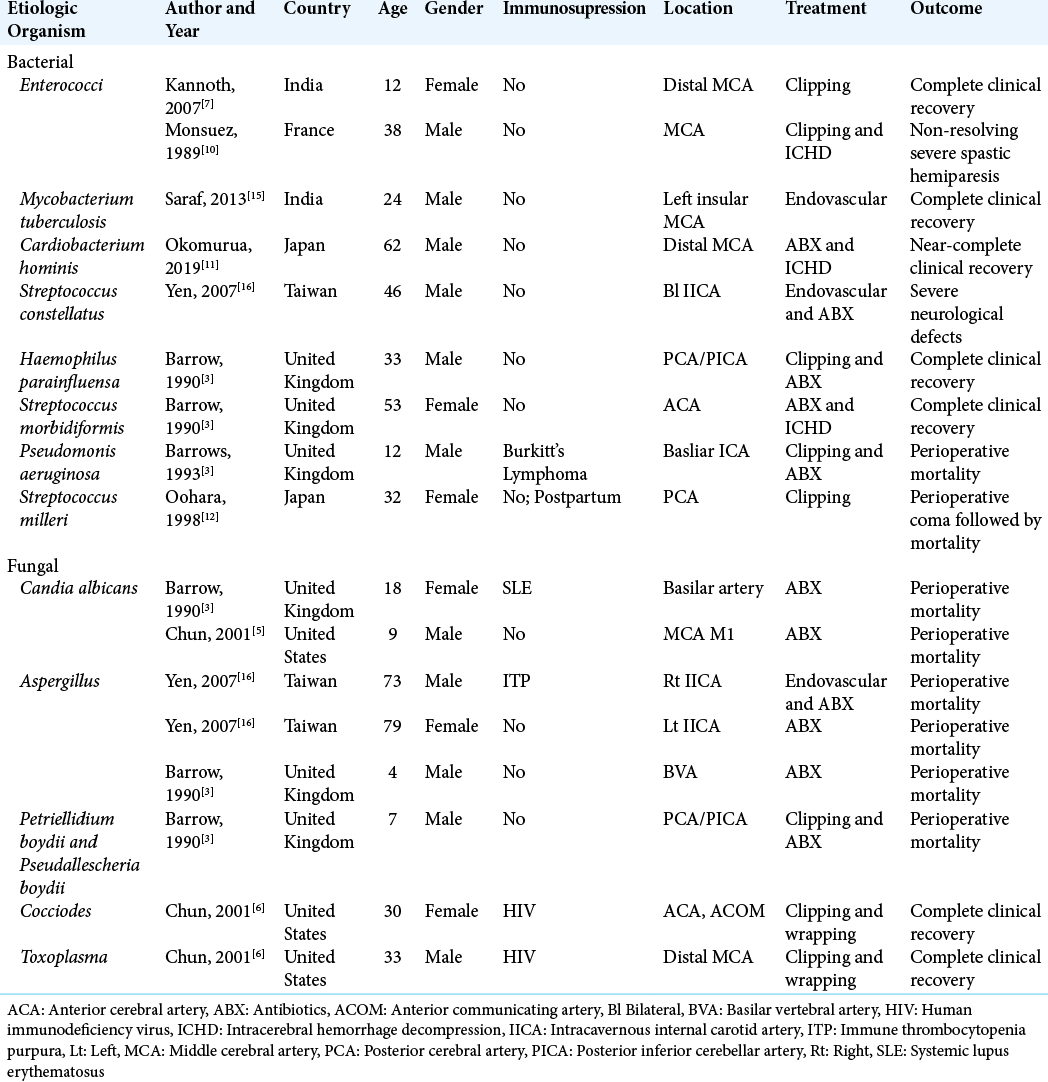
With regard to the etiologic organism, mycotic aneurysms due to S. anginosus are exceedingly rare, with just one case documented in literature between the years of 1990 and 2020.[
As for treatment of the underlying cardiac pathology, valve surgery for infective endocarditis in IIA patients is typically postponed. Current American Association of Thoracic Surgeon guidelines suggest valve surgery be delayed by 1–2 weeks in the setting of non-hemorrhagic stroke and 3–4 weeks in patients with hemorrhagic strokes. In patients with severe neurologic damage such as the patient in this case, it is recommended cardiovascular surgery be delayed until potential for neurological recovery is established.[
Due to the rarity of ruptured mycotic aneurysms, little data are available regarding long-term care and outcomes of ruptured IIAs. Unruptured IIA carry a better prognosis than ruptured IIA however the degree of favorability is uncertain.[
CONCLUSION
IIAs, sometimes referred to as cerebral mycotic aneurysms, are an uncommon but feared complication of bacterial endocarditis. Rupture of an IIA carries devastating neurologic consequences with up to 80% mortality. Even with timely repair of ruptured IIA, there is still a 20–60% likelihood of progressive or permanent neurological deficits. A well-coordinated, multidisciplinary approach to care for a patient with ruptured IIA resulting from infectious endocarditis can yield favorable long-term results.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Ando K, Hasegawa H, Kikuchi B, Saito S, On J, Shibuya K. Treatment strategies for infectious intracranial aneurysms: Report of three cases and review of the literature. Neurol Med Chir (Tokyo). 2019. 59: 344-50
2. Aspoas AR, De Viluers JC. Bacterial intracranial aneurysms. Br J Neurosurg. 1993. 7: 367-76
3. Barrow DL, Prats AR. Infectious intracranial aneurysms: comparison of groups with and without endocarditis. Neurosurgery. 1990. 27: 562-72
4. Chalouhi N, Hoh BL, Hasan D. Review of cerebral aneurysm formation, growth, and rupture. Stroke. 2013. 44: 3613-22
5. Chun JY, Smith W, Halbach VV, Higashida RT, Wilson CB, Lawton MT. Current multimodality management of infectious intracranial aneurysms. Neurosurgery. 2001. 48: 1203-14
6. Ducruet AF, Hickman ZL, Zacharia BE, Narula R, Grobelny BT, Gorski J. Intracranial infectious aneurysms: A comprehensive review. Neurosurg Rev. 2009. 33: 37
7. Kannoth S, Iyer R, Thomas SV, Furtado SV, Rajesh BJ, Kesavadas C. Intracranial infectious aneurysm: Presentation, management and outcome. J Neurol Sci. 2007. 256: 3-9
8. Kuo I, Long T, Nguyen N, Chaudry B, Karp M, Sanossian N. Ruptured intracranial mycotic aneurysm in infective endocarditis: A natural history. Case Rep Med. 2010. 2010: 168408
9. Mincheff TV, Cooler AW. Ruptured mycotic aneurysm presenting initially with bacterial meningitis. Am Surg. 2008. 74: 73-5
10. Monsuez JJ, Vittecoq D, Rosenbaum A, Goujon C, Wolff M, Witchitz S. Prognosis of ruptured intracranial mycotic aneurysms: A review of 12 cases. Eur Heart J. 1989. 10: 821-5
11. Okumura E, Tsurukiri J, Yamanaka H, Nakagawa Y, Ootsuka K, Tanaka Y. Intracranial Hemorrhaging Following Cardiobacterium hominis endocarditis. Int Med. 2019. 58: 1361-5
12. Oohara K, Yamazaki T, Kanou H, Kobayashi A. Infective endocarditis complicated by mycotic cerebral aneurysm: Two case reports of women in the peripartum period. Eur J Cardiothorac Surg. 1998. 14: 533-5
13. Peters PJ, Harrison T, Lennox JL. A dangerous dilemma: Management of infectious intracranial aneurysms complicating endocarditis. Lancet Infect Dis. 2006. 6: 742-8
14. Pettersson GB, Hussain ST. Current AATS guidelines on surgical treatment of infective endocarditis. Ann Cardiothorac Surg. 2019. 8: 630-44
15. Saraf R, Limaye U. Ruptured intracranial tubercular infectious aneurysm secondary to a tuberculoma and its endovascular management. Br J Neurosurg. 2013. 27: 243-5
16. Yen PS, Teo BT, Chen SC, Chiu TL. Endovascular treatment for bilateral mycotic intracavernous carotid aneurysms: Case report and review of the literature. J Neurosurg. 2007. 107: 868-72
17. Yuan SM, Wang GF. Cerebral mycotic aneurysm as a consequence of infective endocarditis: A literature review. Cor Vasa. 2017. 59: e257-65
18. Zanaty M, Chalouhi N, Starke RM, Tjoumakaris S, Gonzalez LF, Hasan D. Endovascular treatment of cerebral mycotic aneurysm: A review of the literature and single center experience. Biomed Res Int. 2013. 2013: 151643