- Department of Neurosurgery, Dharmais National Cancer Hospital, West Jakarta, Indonesia,
- Department of Neurology, Dharmais National Cancer Hospital, West Jakarta, Indonesia,
- Department of Anatomical Pathology, Dharmais National Cancer Hospital, West Jakarta, Indonesia,
- Department of Neurosurgery, Universitas Padjadjaran Facultas Kedokteran, Jawa Barat, Indonesia.
Correspondence Address:
Ahmad Faried
Department of Neurosurgery, Universitas Padjadjaran Facultas Kedokteran, Jawa Barat, Indonesia.
DOI:10.25259/SNI_370_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Muhammad Firdaus1, Arwinder S. Gill1, Rini Andriani2, Dian Cahyanti3, Maria R. Yunti3, Ahmad Faried4. A rare cystic lymphoplasmacyte-rich meningioma: A case report and review of the literature. 18-Nov-2020;11:391
How to cite this URL: Muhammad Firdaus1, Arwinder S. Gill1, Rini Andriani2, Dian Cahyanti3, Maria R. Yunti3, Ahmad Faried4. A rare cystic lymphoplasmacyte-rich meningioma: A case report and review of the literature. 18-Nov-2020;11:391. Available from: https://surgicalneurologyint.com/surgicalint-articles/10394/
Abstract
Background: Meningiomas are common central nervous system neoplasms, accounts for 30% of all primary intracranial neoplasms; the occurrence of meningiomas with cystic lesions is an exceptionally rare. Lymphoplasmacyte-rich meningioma (LPRM) is a rare pathological entity belong to the World Health Organization Grade I meningiomas. LPRM is characterized by abundant lymphoplasmacytic infiltrates which over-shadow the underlying meningothelial component.
Case Description: A 42-year-old male was admitted to our hospital with a chronic headache for about 3 weeks prior to admission. His symptoms worsen, and subsequently, he experienced left extremities weakness about 1 week before admission. His brain magnetic resonance imaging revealed an irregular and heterogeneously enhancing solid lesion with intratumoral cystic changes at the temporal lobe. A gross total resection was performed; pathological examination revealed a cystic LPRM.
Conclusion: This rare variant of meningioma is a benign tumor entity featured with massive inflammatory cell infiltration and often less proportion of meningothelial elements. Surgical resection remains the treatment of choice. This is the first report regarding cystic LPRM from Indonesia; we also summarized relevant literature upto-date, May 2020, reported LPRM cases.
Keywords: Cystic lymphoplasmacyte-rich meningioma, Extremely rare meningioma variant case, Histopathology, Treatment, Up-date literature review
INTRODUCTION
Meningioma is a common neoplasm of the central nervous system, accounting for 30% of all primary intracranial neoplasms.[
CASE REPORT
A 42-year-old male was admitted to our hospital with chronic headache since 3 weeks before admission. His headache progressively worsened and accompanied with weakness on his left side since 1 week before admission. His weakness was not associated with sensory deficits. He denied any history of trauma, seizures, vomiting, fever, or other associated symptoms. There was a history of alcohol-tobacco consumption >10 years. Neurological examination revealed a grade 4/5 motor strength on both left upper- and lower-limb, other examinations were within normal limits. A complete blood count, erythrocyte sedimentation rate (ESR), coagulation profile, liver-kidney chemistries, and urinalysis were within normal limits.
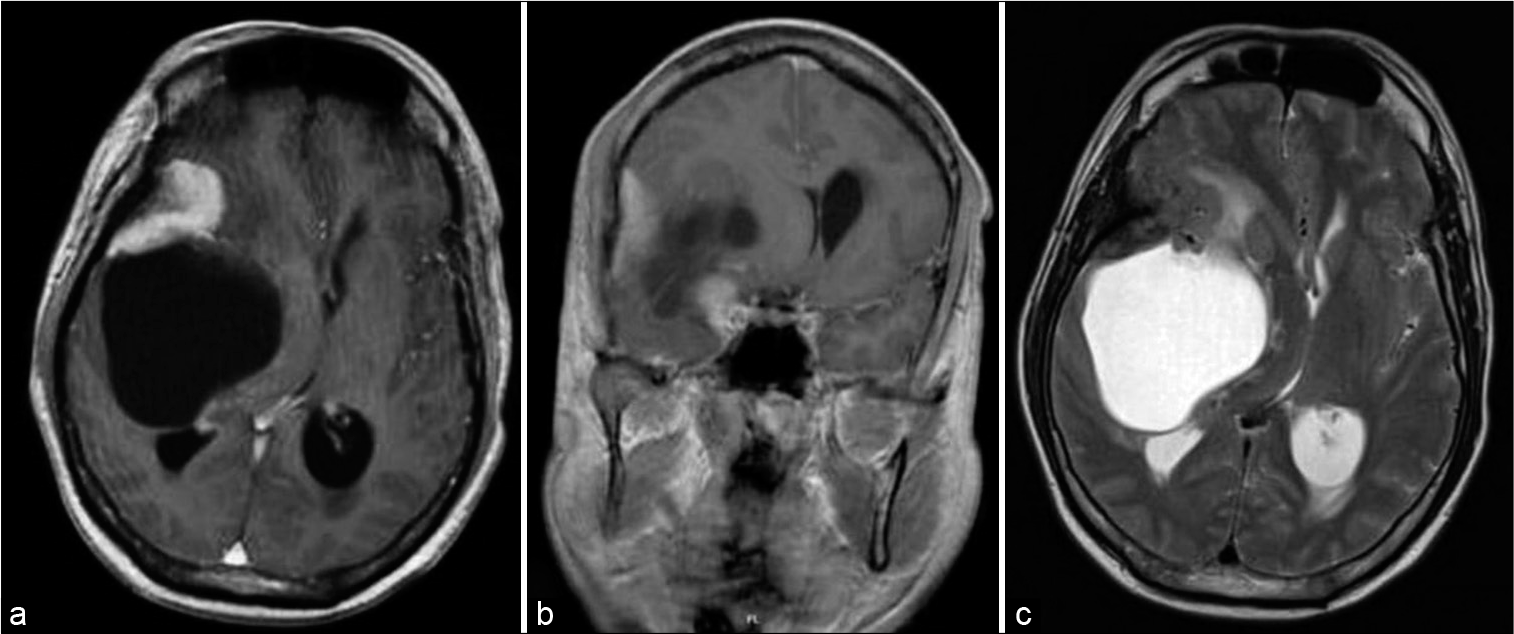
Brain magnetic resonance imaging (MRI) revealed an irregular and heterogeneously enhancing solid lesion at the right temporoparietal region, with intratumoral cystic changes, a broad attachment on the dura, and perifocal brain edema resulting in midline shift to the contralateral side [
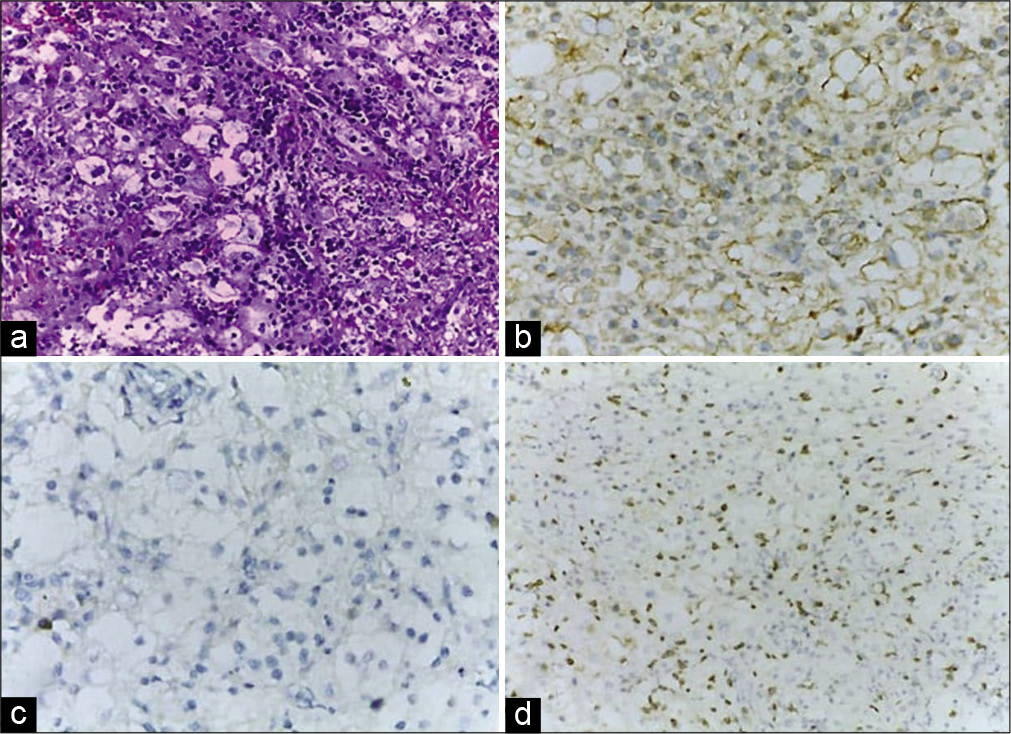
The pathological examination revealed the proliferation of neoplastic epithelial cells with eosinophilic cytoplasm within solid nests, with surrounding inflammatory infiltrates, rich in lymphocytes, and plasma cells. The pathological examination also revealed the formation of lymphoid follicles and foci of fibrosis. The tumor tissue was positively stained for vimentin, but negative for glial fibrillary acidic protein. The inflammatory infiltrates were mostly stained with CD3 [
Figure 2:
Microscopic examination revealed the proliferation of neoplastic meningothelial cells with pale eosinophilic cytoplasm forming solid nests, associated with a dense chronic inflammatory infiltrate rich in lymphocytes and some plasma cells (a) (H&E, ×20). Both tumor cells and lymphocytes are positive with vimentin (b) (×20). Negative glial fibrillary acidic protein in tumor cells excludes the diagnosis of glioma with xanthomatous changes (c) (×40). CD3 staining in lymphocytes dispersed between tumor cells (d) (×20).
DISCUSSION
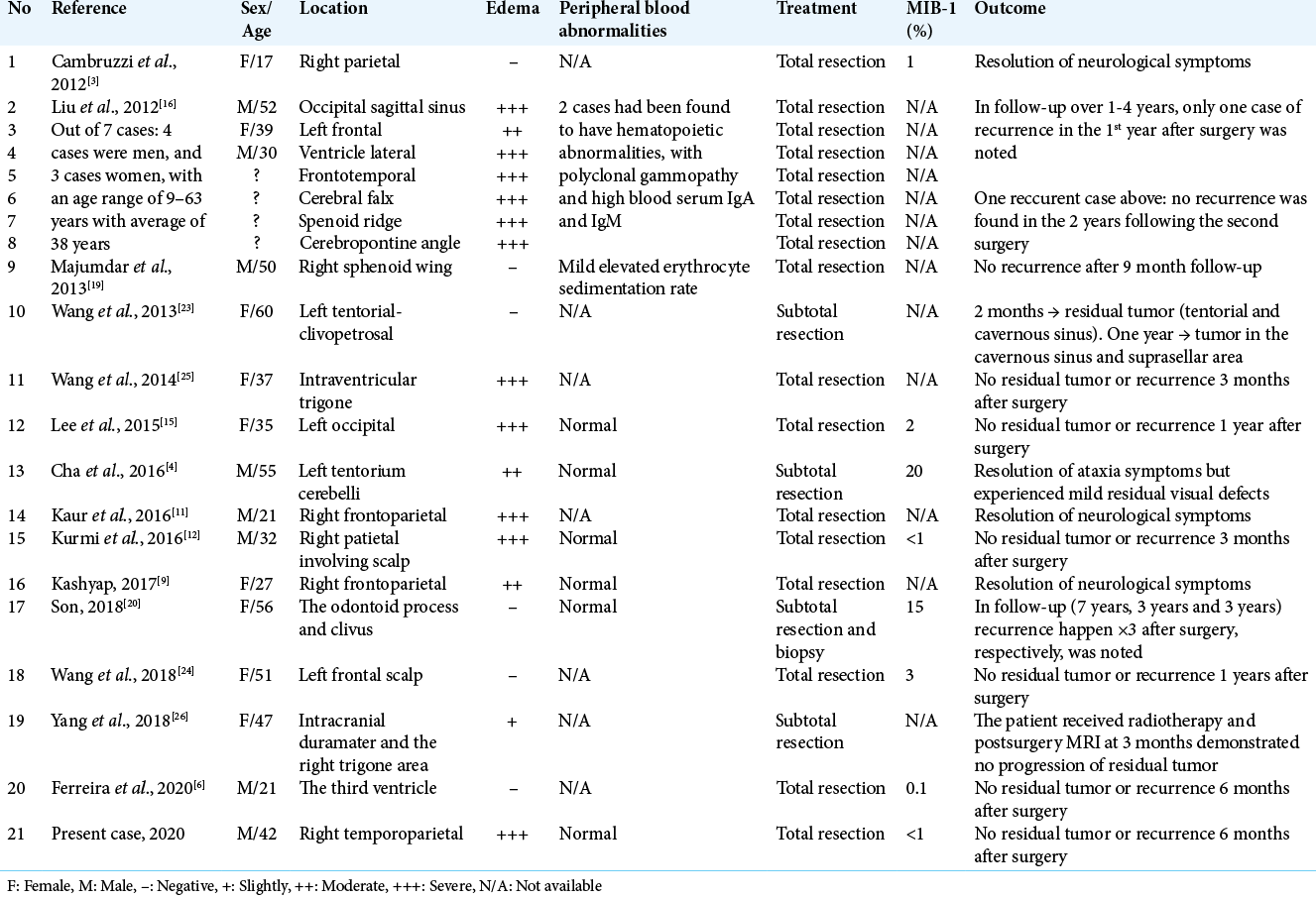
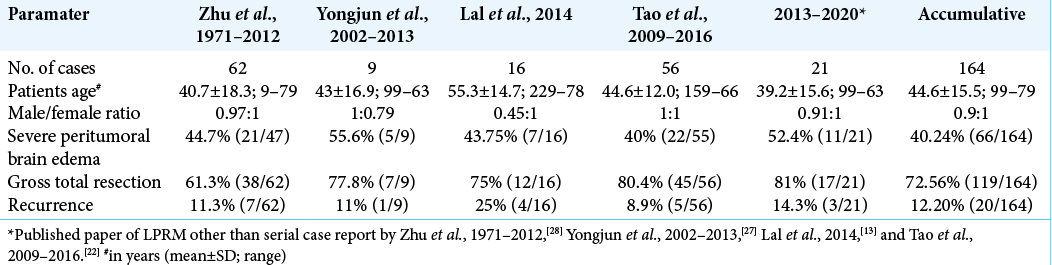
LRPM, a rare WHO grade I subtype, usually occurs in young and middle-aged patients without sex predominance; in our case, it is even extremely rare since it occurred with a cystic lesion that has been only ten reported in the literature (became 11 with our case).[
Here, we reported – to the best of our knowledge – the first LPRM case from Indonesia and reviewed relevant up-date literatures summarized LPRM cases (using pathological examination methods); the summary of previously documented LPRM cases [
From all 21 patients, MRI and computed tomography scan images were available in all patients. Most of the lesions exhibited hypo-to-isointense signal on T1-weighted images and hypo-iso- to high-intense signal on T2-weighted images, usually with homogenous enhancement after gadolinium administration; a classical dural tail sign was observed in some case. Most cases were diagnosed as meningiomas but not LPRM before surgery. In addition, 71.4% (15/21; slightly-edema 1, moderate-edema 3, and severe-edema 11) of the lesions exhibited peritumoral edema with peritumoral or intratumoral cystic changes were observed in 52.4% (11/21) of the patients. Preoperative MRI diagnosis of meningioma was made in seven cases, an aggressive meningioma was in three cases, inflammatory granuloma in two cases, malignant brain tumor in two cases, metastatic tumor in two cases, lymphoma in one case, sinus thrombosis in one case, craniopharyngioma in one case, and glioma in one case. One case had an extensive left tentorial-petroclival extra-axial tumor that was irregular in shape and compressed the brain stem; subtotal resection was then performed followed by gamma knife surgery (GKS) at first to treat the residual tumor.[
There are various of differential diagnoses for these types of cortical mass and biological abnormalities such as idiopathic hypertrophic pachymeningitis, giant lymph node hyperplasia, plasma cell granuloma, multiple myeloma, chordoid meningioma, solitary plasmacytoma, sinus histiocytosis, and lymphomatoid granulomatosis.[
The significance of blood abnormalities found in LPRM remains elusive. Stam et al. concluded that the plasma cell infiltrates were not tumoral in origin due to the abundant production of almost all classes of immunoglobulins.[
Based on the Simpson grading criteria, 17 of 21 cases had total tumor resection (Simpson Grade I or II), while four cases had subtotal resection or biopsy (Simpson Grade III or IV) since a complete resection was not possible.[
To date, 164 LPRM cases, including 11 cystic LPRM from previous case reports and retrospective case series, have been reported. Zhu et al.[
LPRM occurs at a higher rate in young and middle-aged patients without sex predominance, which differs from other types of meningiomas.[
CONCLUSION
Cystic LPRM is an extremely rare benign variant of intracranial meningioma that occurs at a higher rate in young and middle-aged patients without sex predominance with a low tendency of recurrence, mainly in the convexity, featured with a massive inflammatory cells infiltration and often a less proportion of meningothelial components. A definitive diagnosis was possible only through a histopathological examination, along with a good communication between the surgeon and the pathologist. Total surgical resection remains a primary goal of treatment.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Dr. Ahmad Faried received a research grant from Universitas Padjadjaran, Bandung and supported by the Grants-inAid from Indonesian Ministry of Research and Technology (National Research and Innovation Agency) 16/E1/ KPT/2020 for basic research.
Conflicts of interest
There are no conflicts of interest.
References
1. Banerjee AK, Blackwood W. A subfrontal tumour with the features of plasmocytoma and meningioma. Acta Neuropathol. 1971. 18: 84-8
2. Bruno MC, Ginguené C, Santangelo M, Panagiotopoulos K, Piscopo GA, Tortora F. Lymphoplasmacyte rich meningioma. A case report and review of the literature. J Neurosurg Sci. 2004. 48: 117-24
3. Cambruzzi E, da Costa de Souza TA, Silveira LC, dos Santos Moreira CF. Lymphoplasmacyte-rich meningioma: A case report of a rare neoplasm. J Bras Patol Med Lab. 2012. 48: 223-7
4. Cha YJ, Lee SK, Chang JH, Kim SH. Report of a rare case of atypical lymphoplasmacyte-rich meningioma in the tentorium mimicking idiopathic hypertrophic pachymeningitis. Brain Tumor Pathol. 2016. 33: 216-21
5. Claus EB, Bondy ML, Schildkraut JM, Wiemels JL, Wrensch M, Black PM. Epidemiology of intracranial meningiomas. Neurosurgery. 2005. 57: 1088-95
6. Ferreira MP, Cambruzzi E, Martins OG, Gago G, Vial AD. Lymphoplasmacyte-rich meningioma of 3rd ventricle: Case report. Arq Bras Neurocir. 2020. 39: 149-53
7. Fortuna A, Ferrante L, Acqui M, Guglielmi G, Mastronardi L. Cystic meningiomas. Acta Neurochir (Wien). 1988. 90: 23-30
8. Go Ko, Lee K, Heo W, Lee YS, Park YS, Kim SK. Cystic meningiomas: Correlation between radiologic and histopathologic features. Brain Tumor Res Treat. 2018. 6: 13-21
9. Karshyap A, Mukherjee T, Chauhan RD. Lymphoplsdmacyte-rich meningioma-a rare case report and review of the literature. World J Surg Med Radiat Oncol. 2017. 6: 17-21
10. Katayama S, Fukuhara T, Wani T, Namba S, Yamadori I. Cystic lymphoplasmacyte-rich meningioma-case report. Neurol Med Chir (Tokyo). 1997. 37: 275-8
11. Kaur M, Dalal V, Sharma KC, Singh A. Lypmhoplasmacyte rich meningioma-a rare morphological variant of meningioma. Br J Med Pract. 2016. 9: a905
12. Kurmi DJ, Sharma A, Mittal RS, Singhvi S. Lymphoplasmacyte-rich meningioma with invasion of bone: A case report and review of literature. Asian J Neurosurg. 2016. 11: 448
13. Lal A, Dahiya S, Gonzales M, Hiniker A, Prayson R, Kleinschmidt-DeMasters BK, Perry A. IgG4 overexpression is rare in meningiomas with a prominent inflammatory component: A review of 16 cases. Brain Pathol. 2014. 24: 352-9
14. Lee KJ, Joo WI, Rha HK, Park HK, Chough JK, Hong YK. Peritumoral brain edema in meningiomas: Correlations between magnetic resonance imaging, angiography, and pathology. Surg Neurol. 2008. 69: 350-5
15. Lee MY, Ahn K, Lee YS, Jeun SS. Neuroimaging and clinicopathologic findings of lymphoplasmacyte-rich meningioma, mimicking malignancy: Case report. Investig Magn Reson Imaging. 2015. 19: 62-6
16. Liu JL, Zhou JL, Ma YH, Dong C. An analysis of the magnetic resonance imaging and pathology of intracal lymphoplasmacyte-rich meningioma. Eur J Radiol. 2012. 81: 968-73
17. Loh JK, Hwang SL, Tsai KB, Kwan AL, Howng SL. Sphenoid ridge lymphoplasmacyte-rich meningioma. J Formos Med Assoc. 2006. 105: 594-8
18. Louis DN, Perry A, Reifenberger G, von Deimling A, Branger DF, Cavenee WK. The 2016 World Health Organization classification of tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016. 131: 803-20
19. Majumdar K, Saran RK, Chaurasia PK, Tyagi I, Singh D. Sphenoid wing lymphoplasmacyte-rich meningioma with occasional emperipolesis closely simulating an intracranial Rosai-Dorfman disease: A diagnostic dilemma. Clin Neuropathol. 2013. 32: 122-7
20. Son HJ, Yu IK, Kim SM. Lymphoplasmacyte-rich meningioma with atypical angiomatous feature and an increased deposition of IGg4-positive plasma cells: An unusual case report. Int J Surg Pathol. 2018. 26: 93-7
21. Stam FC, van Alphen HS, Boorsma DM. Meningioma with conspicuous plasma cell components. A histopathological and immunohistochemical study. Acta Neuropathol. 1980. 49: 241-3
22. Tao X, Wang K, Dong J, Hou Z, Wu Z, Zhang J. Clinical, radiologic, and pathologic features of 56 cases of intracranial lymphoplasmacyte-rich meningioma. World Neurosurg. 2017. 106: 152-64
23. Wang WH, Lee CC, Lin SC, Guo WY, Ho DM, Chen MH. Gamma knife radiosurgery for lymphoplasmacyte-rich meningioma. Clin Neurol Neurosurg. 2013. 115: 1110-3
24. Wang Y, Teng Y, Xu H, Li Y. Primary intraosseous lymphoplasmacyte-rich meningioma. World Neurosurg. 2018. 109: 291-3
25. Wang YB, Wang WJ, Xu SB, Xu BF, Yu Y, Ma H. Intraventricular lymphoplasmacyte-rich meningioma: A case report. Turk Neurosurg. 2014. 24: 958-62
26. Yang X, Le J, Hu X, Zhang Y, Liu J. Lymphoplasmacyte-rich meningioma involving the whole intracranial dura mater. Neurology. 2018. 90: 934-5
27. Yongjun L, Xin L, Qiu S, Jun-Lin Z. Imaging findings and clinical features of intracal lymphoplasmacyte-rich meningioma. J Craniofac Surg. 2015. 26: e132-7
28. Zhu HD, Xie Q, Gong Y, Mao Y, Zhong P, Hang FP. Lymphoplasmacyte-rich meningioma: Our experience with 19 cases and a systematic literature review. Int J Clin Exp Med. 2013. 6: 504-15