- Department of Neurosurgery, Leeds General Infirmary, Leeds, United Kingdom.
Correspondence Address:
Sarvesh Kutty, Department of Neurosurgery, Leeds General Infirmary, Leeds, United Kingdom.
DOI:10.25259/SNI_731_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sarvesh Kutty. A summary of common grading systems used in neurosurgical practice. 28-Oct-2022;13:497
How to cite this URL: Sarvesh Kutty. A summary of common grading systems used in neurosurgical practice. 28-Oct-2022;13:497. Available from: https://surgicalneurologyint.com/surgicalint-articles/11957/
Abstract
Background: Grading and scoring systems are routinely used across various specialties in medicine and surgery. They help us assess the severity of disease and often guide management as well. In addition, grading systems allow us to prognosticate and gauge outcomes. Neurosurgeons also utilize an array of scores and grading systems. This article aims to collate some of the common grading systems used in neurosurgical practice to be utilized as an easy reference especially for junior doctors and other health-care providers working in this field.
Methods: An initial literature search was carried out to look at the grading systems in use. These were then distilled down to the ones that are frequently used in clinical neurosurgical practice based on my own experience as a doctor working in a tertiary neurosurgical unit. Neuro-oncology scoring systems were excluded from the study.
Results: Grading systems are grouped based on the area of neurosurgical practice they fall into such as cranial, vascular, spinal, and miscellaneous. A brief description of each grading system is provided and the conditions when they can be used in a tabular format. Discussion on the advantages and disadvantages of each grading system is not included in the study.
Conclusion: The list of grading systems in this article is not exhaustive. To the best of my knowledge, there seems to be no recent article, which summarizes them concisely. I hope that this summary will benefit the neurosurgical community and wider audience.
Keywords: Grading systems, Neurosurgery, Scores
INTRODUCTION
There are numerous grading systems used in neurosurgery. This article aims to give a brief summary of some of the commonly encountered grading systems in practice. For ease of use, tables are provided for each of them. Although there are various articles on the individual grading systems listed below, I believe that this concise tabular format will benefit clinicians working in neurosurgery. The grading systems have been classified into the subspecialty of neurosurgical practice they fall into. Neuro-oncology scoring systems were excluded from this study due to the complexity of the current guidelines.
CRANIAL NEUROSURGERY
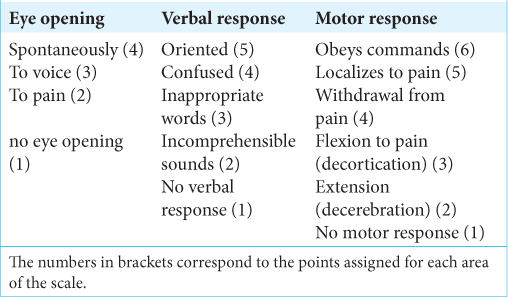
Glasgow coma scale (GCS)
Professor Teasdale and Jennett first published the GCS in 1974 in the Lancet.[
Pediatric GCS
The pediatric GCS [
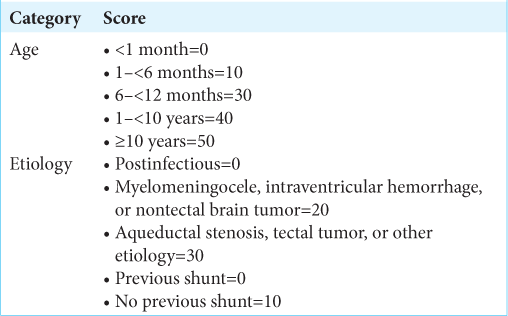
Endoscopic third ventriculostomy (ETV) success score
An ETV is a procedure done mainly for obstructive hydrocephalus (noncommunicating). However, its use is not limited to this condition. The procedure involves making a hole in the floor of the third ventricle to allow cerebrospinal fluid to flow.[
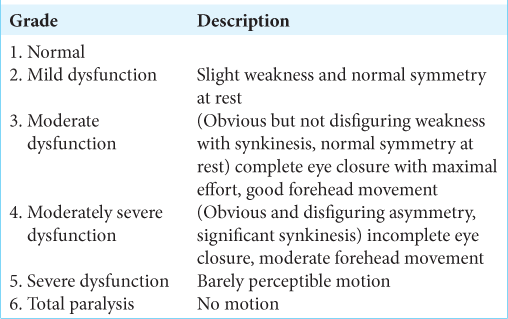
House-Brackmann classification for facial nerve palsy
This is a scoring system proposed by Dr. House and Brackmann in 1985. The purpose of this is to assess the severity of facial nerve palsy.[
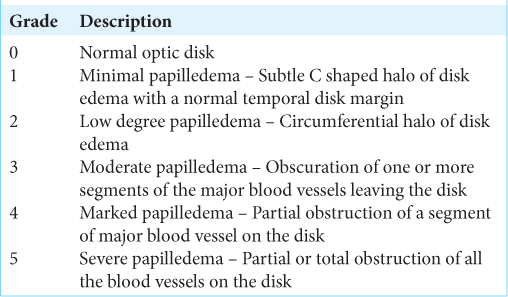
Frisen scale for papilledema
The Frisen and modified Frisen scale describes the grade of optic disk swelling in conditions with raised intracranial pressure.[
Grading for diffuse axonal injury (DAI)
Adams et al. described in 1989 a grading system for DAI based on histology of anatomic distribution of cerebral hemorrhage.[
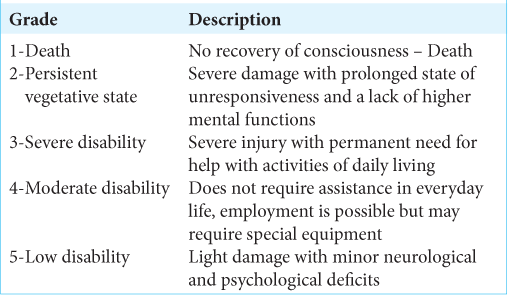
The Glasgow outcome scale (GOS)
The GOS aims to assess outcome after head injury.[
VASCULAR NEUROSURGERY
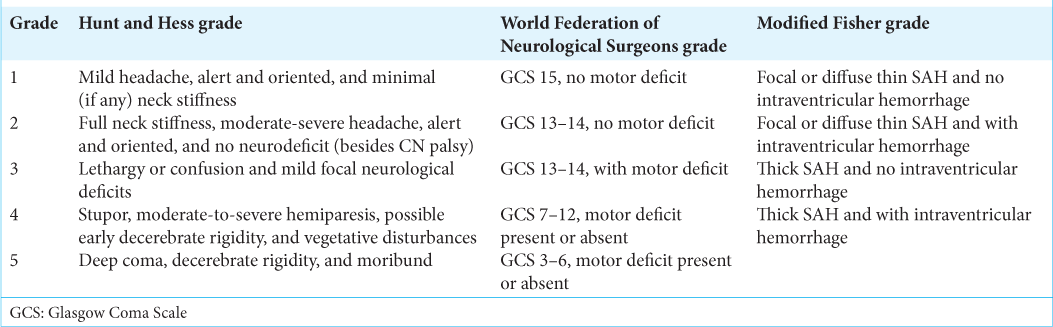
Grading systems for subarachnoid hemorrhage (SAH)
There are three main grading systems used for aneurysmal SAH. The Hunt and Hess classification quantifies the severity of SAH to predict mortality.[
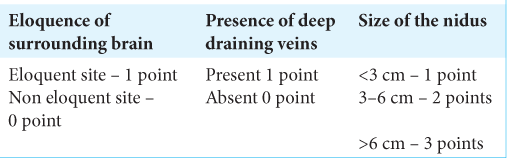
Spetzler-Martin grade for arteriovenous malformation (AVM)
The Spetzler-Martin grading system [
Alberta stroke program early CT score (ASPECTS)
The ASPECTS is a scoring system based on 10 points and is used to predict outcome of middle cerebral artery stroke.[
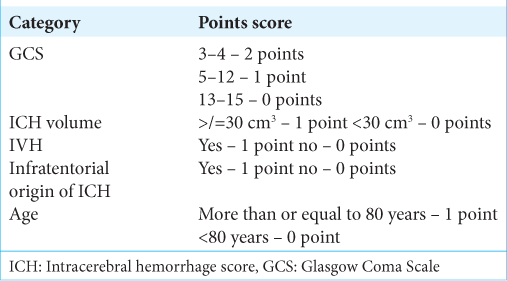
The intracerebral hemorrhage score (ICH)
The ICH score is used to help grade patients with ICH.[
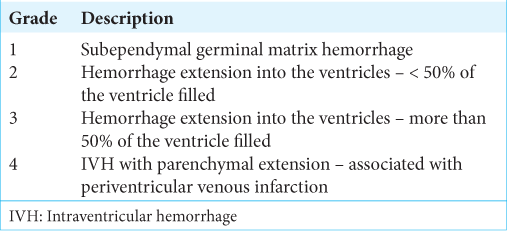
Papile-Burstein classification for IVH
Another useful and commonly used grading system in neurosurgical patients is a grading system used for IVH proposed by Papile et al.[
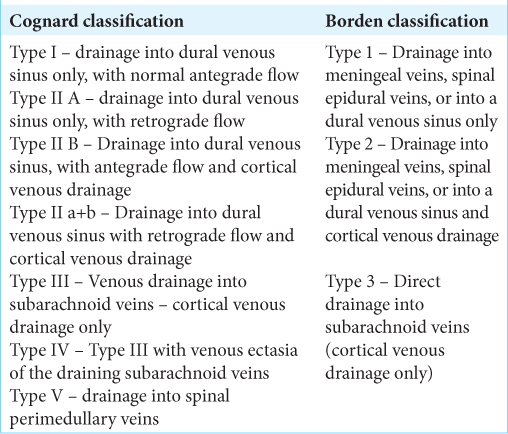
Classification systems for dural arteriovenous fistula (DAVF)
Borden et al. classification for DAVF was proposed in 1995.[
NIH stroke scale/score (NIHSS)
The NIHSS aims to describe the severity of ischemic stroke, with a score of 0–42 being assigned to patients. Higher scores are associated with greater stroke severity.[
SPINAL NEUROSURGERY AND MISCELLANEOUS
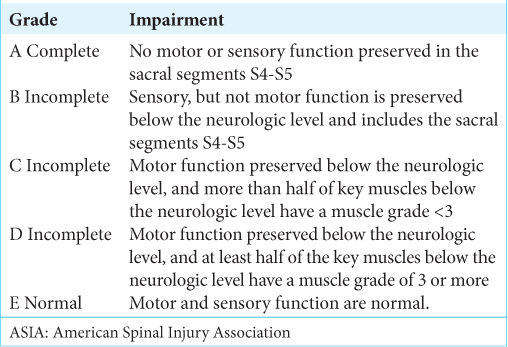
American spinal injury association (ASIA) impairment scale and grade in spinal cord injury
This is a grading system consisting of five grades, allowing clinicians to assess the severity of spinal cord injury.[
Medical research council grading system for muscle strength
This is a commonly used grading system in neurosurgical practice to assess patient’s muscle strength. Much like the GCS, it serves as a reliable tool for nurses and doctors to utilize and regularly document the clinical status of the patient. In addition, it can guide treatment, assess response to treatment, and aid in prognostication. It consists of six grades (0–5), as depicted in
Karnofsky clinical performance status
The Karnofsky clinical performance status is useful in assessing patient’s functional status and suitability for treatment such as chemotherapy.[
Modified Rankin scale
The modified Rankin scale is used to assess outcome after stroke. It is also useful to assess rehabilitation requirements[
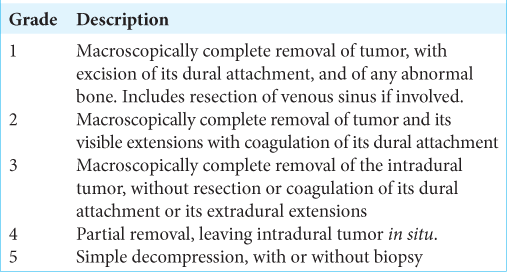
Simpson grade of meningioma resection
The Simpson grade for meningioma resection aims to correlate the degree of surgical resection with the likelihood of meningioma recurrence.[
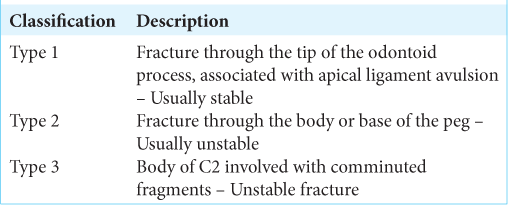
Anderson and D’Alonzo classification of odontoid process fracture
Anderson and D’Alonzo described an important and commonly used classification system for odontoid process fractures.[
Galassi classification of arachnoid cyst
Galassi et al. described a classification system for middle cranial fossa arachnoid cysts in 1982.[
DISCUSSION
There are a vast number of common and obscure grading systems in clinical and research practice. Sifting out the most relevant one for the clinical scenario is not an easy task for clinicians. Only by incorporating them into routine practice that one realizes that there is no single “gold standard” scale, rather many scales albeit with different properties. Based on your clinical requirement, one should choose the most appropriate grading system. The grading system should have the ability to be incorporated seamlessly into routine clinical use. Moreover, it should be reliable, repeatable, and provide a valid measurement for the specific outcome.
Over the past two decades, there has been an explosion of psychometric methods using statistical techniques in an attempt to provide strength to the measurements obtained from grading systems.[
Tremendous amount of work and expertise have gone into the creation of grading systems over the years and this article is an attempt to acknowledge these contributions to health measurements. In the process, I would like to draw the attention of clinicians to the nuances, limitations, and benefits of such systems, as pointed out by Massof[
CONCLUSION
Conclusions drawn from the various measurements used in grading systems dictate patient care. Some of them such as GCS scoring have immediate outcomes, whereas many disability ratings have significant long-term impact including health-care expenditure, clinical guidelines, and even policy-making. Therefore, it is crucial that those of us using them are aware of the validity and quality of the rating scales used in clinical settings. Many variables such as patient perspectives and quality of life indices studies do need stringent measurement criteria as they can change some clinical practices. These facts have been pointed out by studies of rating scales in the field of neurology, where the choice of rating scales had affected the clinical course of diseases such as multiple sclerosis.[
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Adams HP, Davis PH, Leira EC, Chang KC, Bendixen BH, Clarke WR. Baseline NIH stroke scale score strongly predicts outcome after stroke: A report of the trial of org 10172 in acute stroke treatment (TOAST). Neurology. 1999. 53: 126-31
2. Adams JH, Doyle D, Ford I, Gennarelli TA, Graham DI, McLellan DR. Diffuse axonal injury in head injury: Definition, diagnosis and grading. Histopathology. 1989. 15: 49-59
3. Anderson LD, D’Alonzo RT. Fractures of the odontoid process of the axis. J Bone Joint Surg Am. 1974. 56: 1663-74
4. ASIA and ISCoS International Standards Committ. The 2019 revision of the international standards for neurological classification of spinal cord injury (ISNCSCI)-what’s new?. Spinal Cord. 2019. 57: 815-7
5. Association American Spinal Inju, editors. Standards for Neurological Classification of Spinal Injury Patients. Chicago, IL: American Spinal Injury Association; 1982. p.
6. Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS study group. Alberta stroke programme early CT score. Lancet. 2000. 355: 1670-4
7. Borden JA, Wu JK, Shucart WA. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg. 1995. 82: 166-79
8. Borgialli DA, Mahajan P, Hoyle JD, Powell EC, Nadel FM, Tunik MG. Performance of the pediatric glasgow coma scale score in the evaluation of children with blunt head trauma. Acad Emerg Med. 2016. 23: 878-84
9. Chotai S, Schwartz TH. The simpson grading: Is it still valid?. Cancers (Basel). 2022. 14: 2007
10. Cognard C, Gobin YP, Pierot L, Bailly AL, Houdart E, Casasco A. Cerebral dural arteriovenous fistulas: Clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995. 194: 671-80
11. Compston A. Aids to the investigation of peripheral nerve injuries. Medical research council: Nerve injuries research committee, His majesty’s stationery office: 1942; p. 48 (iii) and 74 figures and 7 diagrams; with aids to the examination of the peripheral nervous system. By michael O’brien for the guarantors of brain. Saunders Elsevier: 2010; pp. [8] 64 and 94 figures. Brain. 2010. 133: 2838-44
12. Das S, Montemurro N, Ashfaq M, Ghosh D, Sarker AC, Khan AH. Resolution of papilledema following ventriculoperitoneal shunt or endoscopic third ventriculostomy for obstructive hydrocephalus: A pilot study. Medicina (Kaunas). 2022. 58: 281
13. Demerdash A, Rocque BG, Johnston J, Rozzelle CJ, Yalcin B, Oskouian R. Endoscopic third ventriculostomy: A historical review. Br J Neurosurg. 2017. 31: 28-32
14. Drake CG, Hunt WE, Sano K, Kassell N, Teasdale G, Pertuiset B. Report of world federation of neurological surgeons committee on a universal subarachnoid haemorrhage grading scale. J Neurosurg. 1988. 68: 985-6
15. Dunn LT. Raised intracranial pressure. J Neurol Neurosurg Psychiatry. 2002. 73: i23-7
16. Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid haemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980. 6: 1-9
17. Frisén L. Swelling of the optic nerve head: A staging scheme. J Neurol Neurosurg Psychiatry. 1982. 45: 13-8
18. Frontera JA, Claassen J, Schmidt JM, Wartenberg KE, Temes R, Connolly ES. Prediction of symptomatic vasospasm after subarachnoid haemorrhage: The modified fisher scale. Neurosurgery. 2006. 59: 21-7
19. Galassi E, Tognetti F, Gaist G, Fagioli L, Frank F, Frank G. CT scan and metrizamide CT cisternography in arachnoid cysts of the middle cranial fossa: Classification and pathophysiological aspects. Surg Neurol. 1982. 17: 363-9
20. Gentry LR. Imaging of closed head injury. Radiology. 1994. 191: 1-17
21. Hemphill JC, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: A simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001. 32: 891-7
22. Hobart J. Rating scales for neurologists. J Neurol Neurosurg Psychiatry. 2003. 74: iv22-6
23. House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985. 93: 146-7
24. Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968. 28: 14-20
25. James HE. Neurologic evaluation and support in the child with an acute brain insult. Pediatr Ann. 1986. 15: 16-22
26. Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975. 1: 480-4
27. Karnofsky DA, Burchenal JH, MacLeod CM, editors. The clinical evaluation of chemotherapeutic agents in cancer. Evaluation of Chemotherapeutic Agents. New York: Columbia University Press; 1949. p. 196-196
28. Kulkarni AV, Drake JM, Mallucci CL, Sgouros S, Roth J, Constantini S. Endoscopic third ventriculostomy in the treatment of childhood hydrocephalus. J Paediatr. 2009. 155: 254-9.e1
29. Lyden P, Brott T, Tilley B, Welch KM, Mascha EJ, Levine S. Improved reliability of the NIH stroke scale using video training. NINDS TPA stroke study group. Stroke. 1994. 25: 2220-6
30. Massof RW. The measurement of vision disability. Optom Vis Sci. 2002. 79: 516-52
31. O’Toole DM, Golden AM. Evaluating cancer patients for rehabilitation potential. West J Med. 1991. 155: 384-7
32. Oya S, Kawai K, Nakatomi H, Saito N. Significance of Simpson grading system in modern meningioma surgery: Integration of the grade with MIB-1 labeling index as a key to predict the recurrence of WHO Grade I meningiomas. J Neurosurg. 2012. 117: 121-8
33. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1,500 gm. J Pediatr. 1978. 92: 529-34
34. Quinn TJ, Dawson J, Walters M. Dr John Rankin; His life, legacy and the 50th anniversary of the Rankin stroke scale. Scott Med J. 2008. 53: 44-7
35. Quinn TJ, Dawson J, Walters MR, Lees KR. Reliability of the modified Rankin scale: A systematic review. Stroke. 2009. 40: 3393-5
36. Rasch G, editors. Probabilistic Models for Some Intelligence and Attainment Tests. Chicago: University of Chicago Press; 1960. p.
37. Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatr. 1957. 20: 22-39
38. Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986. 65: 476-83
39. Strom RG, Botros JA, Refai D, Moran CJ, Cross DT, Chicoine MR. Cranial dural arteriovenous fistulae: Asymptomatic cortical venous drainage portends less aggressive clinical course. Neurosurgery. 2009. 64: 241-7
40. Sun MZ, Oh MC, Safaee M, Kaur G, Parsa AT. Neuroanatomical correlation of the House-Brackmann grading system in the microsurgical treatment of vestibular schwannoma. Neurosurg Focus. 2012. 33: E7
41. Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974. 2: 81-4
42. Van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988. 19: 604-7
43. Wright BD, Linacre JM. Observations are always ordinal: Measurements, however, must be interval. Arch Phys Med Rehabil. 1989. 70: 857-60
44. Zipfel GJ, Shah MN, Refai D, Dacey RG, Derdeyn CP. Cranial dural arteriovenous fistulas: Modification of angiographic classification scales based on new natural history data. Neurosurg Focus. 2009. 26: E14