- Department of Neurosurgery, Prasat Neurological Institute, Bangkok, Thailand.
- Department of Neuroradiology, Prasat Neurological Institute, Bangkok, Thailand.
- Department of Radiology, Bumrungrad International Hospital, Bangkok, Thailand.
Correspondence Address:
Prasert Iampreechakul, Department of Neurosurgery, Prasat Neurological Institute, Bangkok, Thailand.
DOI:10.25259/SNI_11_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Prasert Iampreechakul1, Korrapakc Wangtanaphat1, Sunisa Hangsapruek2, Yodkhwan Wattanasen2, Punjama Lertbutsayanukul2, Somkiet Siriwimonmas3. Acquired Chiari malformation Type I and holocord syringomyelia associated with a high-flow supratentorial fistulous arteriovenous malformations: A case report and literature review. 20-May-2022;13:217
How to cite this URL: Prasert Iampreechakul1, Korrapakc Wangtanaphat1, Sunisa Hangsapruek2, Yodkhwan Wattanasen2, Punjama Lertbutsayanukul2, Somkiet Siriwimonmas3. Acquired Chiari malformation Type I and holocord syringomyelia associated with a high-flow supratentorial fistulous arteriovenous malformations: A case report and literature review. 20-May-2022;13:217. Available from: https://surgicalneurologyint.com/surgicalint-articles/11613/
Abstract
Background: Chiari malformation Type I (CMI) is generally considered a congenital lesion and typically associated with syringomyelia. Acquired CMI or adult Chiari malformation caused by intracranial mass is extremely rare. Brain arteriovenous malformations (AVMs) are characteristically symptomatic due to seizure, intracranial hemorrhage, or neurological deficit. We report an extremely rare case of an acquired CMI and extensive syringomyelia associated with a large supratentorial AVM.
Case Description: A 35-year-old woman was referred to our institute after a diagnosis of CMI and extensive syringomyelia from whole-spine magnetic resonance imaging (MRI) due to complaining of low back pain radiating to the right leg for the past 1 month. She had intermittent headache for 2 years. The patient underwent suboccipital decompression and C1 laminectomy followed by duraplasty. Two months later, she developed severe right-sided sciatic pain and complete right foot drop. Follow-up MRI revealed progressive enlargement of a syrinx cavity at the lower spinal cord and a large right parieto-occipital AVM with markedly dilated cortical draining veins and diffuse engorgement of dural venous sinuses was detected. This AVM supplied mainly by enlarged cortical branches of the right middle cerebral artery and posterior cerebral artery with multiple dural supplies. Endovascular treatment of a high-flow fistulous AVM was successfully performed with N-butyl cyanoacrylate (NBCA) through the hypertrophic branches of the right middle cerebral artery. Four months after embolization, the patient had recovered completely from the right foot drop. Further staged embolization was planned to reduce the size and flow of the AVM before stereotactic radiosurgery. However, the patient was lost to follow-up due to financial reason. One year later, she developed sudden severe headache followed by alteration of conscious due to intraventricular hemorrhage from the AVM, leading to obstructive hydrocephalus requiring cerebrospinal fluid diversion. During a period of 2 years, the patient underwent several staged embolization with NBCA and Onyx. Final cerebral angiography after embolization demonstrated a significant reduction in size and flow of the brain AVM. A control whole-spine MRI revealed a significant reduction in syrinx size. At the end of embolization, the patient had no neurological deficit. However, she had suffered from persistent central neuropathic pain at the right lower extremity. The AVM remnant was further treated by stereotactic radiosurgery.
Conclusion: Increased cerebral venous hypertension secondary to a high-flow supratentorial AVM leading to posterior fossa venous hypertension may play a major role in the pathogenesis of CMI, induced the formation of syringomyelia. Endovascular treatment of brain AVM, the underlying cause of CMI, resulted in a significant reduction of the size of the syrinx. The need for cranial imaging in initial evaluation of cases with adult Chiari malformation is important.
Keywords: Brain arteriovenous malformations, Chiari malformation type I, Foot drop, Holocord syringomyelia, Neuropathic pain
INTRODUCTION
Chiari malformation Type I (CMI) is the most common type of CM and characterized by caudal descent of the cerebellar tonsils exceeding 5 mm below the foramen magnum with no involvement of the brainstem. The onset of symptoms usually occurs during adolescence or adulthood. The predominant clinical symptoms are recurrent headache and cervical pain. CMI is in general a congenital origin related to posterior fossa anomalies and typically associated with syringomyelia, but rarely associated with hydrocephalus. Symptoms of syringomyelia are usually related to spinal cord dysfunction and neuropathic pain.[
Holocord syringomyelia is syrinx involving most or all levels of the spinal cord and patients with extensive syrinx have the potential for more extensive and severe neurological sequelae than those with small syrinxes.[
The authors described an extremely rare case of a large high-flow supratentorial arteriovenous malformation (AVM) causing an acquired CMI with holocord syringomyelia. In our case, CMI was initially misdiagnosed as congenital origin and the patient underwent bony decompression with duraplasty followed by deterioration of the clinical symptoms. Subsequently, a pre-existing supratentorial AVM was discovered accidentally during work up of CMI lesion. The authors also reviewed the patients with brain AVM associated with CMI in the literature.
CASE DESCRIPTION
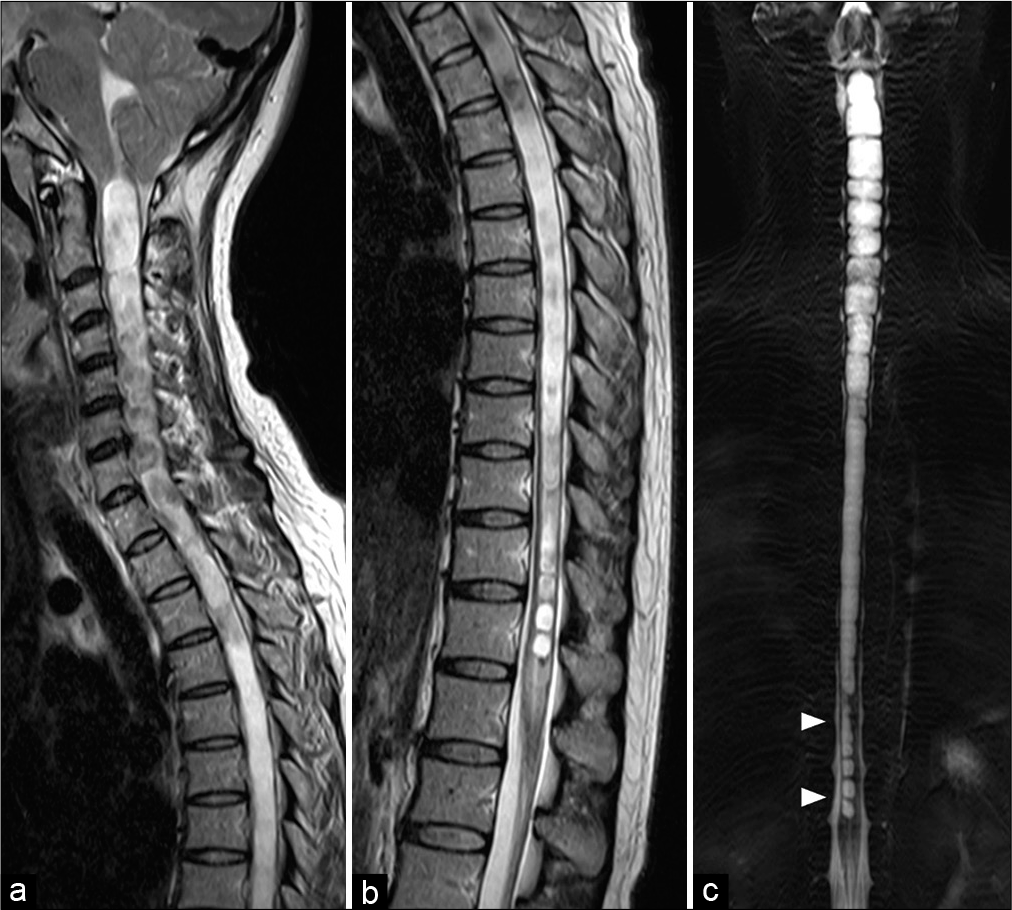
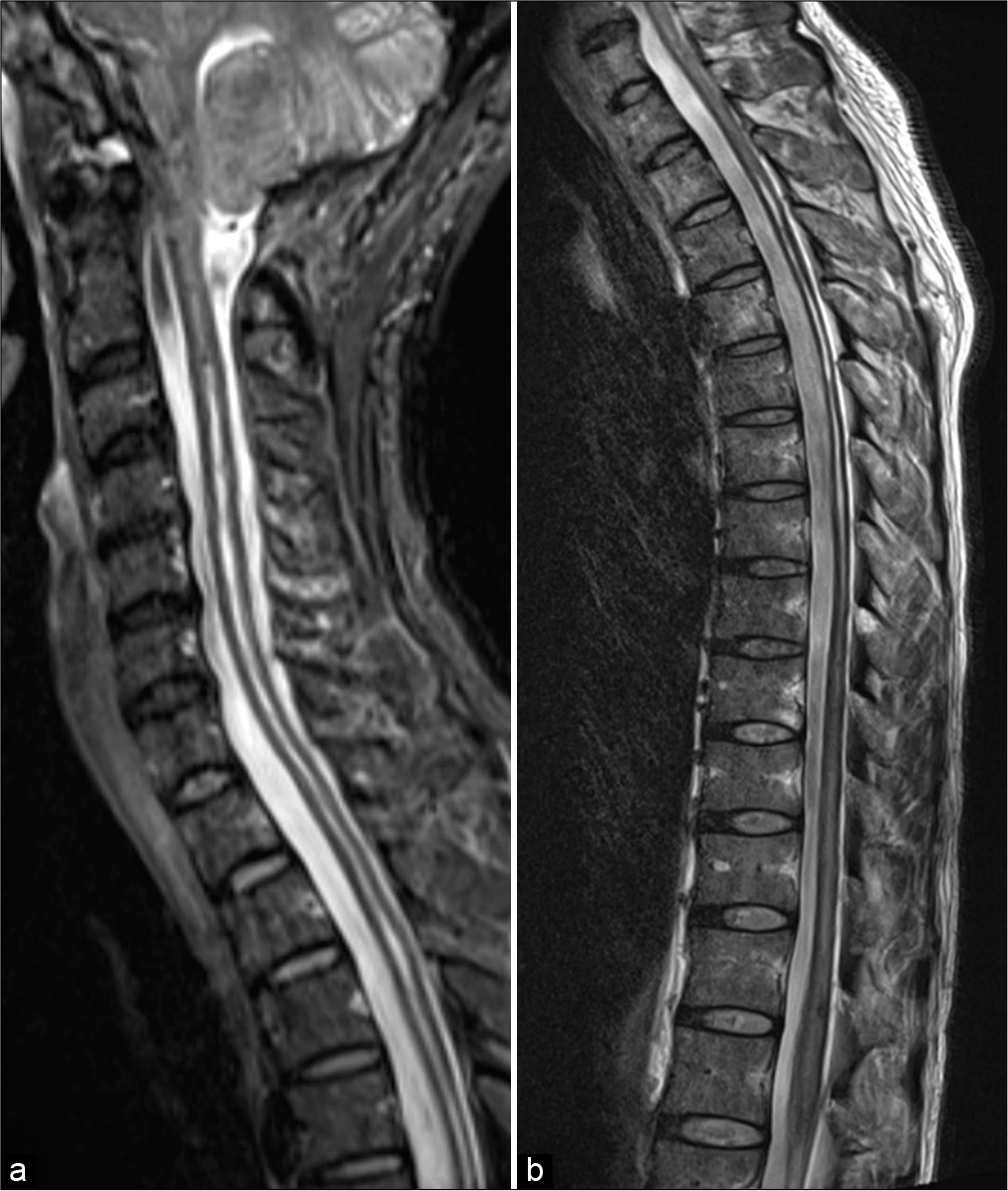
A 35-year-old woman with a medical history of β-thalassemia trait visited the local hospital and complained of intermittent headache for 2 years. She experienced low back pain radiating to the right leg for 1 month. Magnetic resonance imaging (MRI) of the whole spine was obtained and revealed the caudal descent of the cerebellar tonsils through the foramen magnum and a syringomyelic cavity extending from the cervicomedullary junction to the conus medullaris. At the lower spinal cord, syringomyelia affected predominantly on the right side [
Figure 1:
Sagittal T2-weighted magnetic resonance images of (a) cervicothoracic and (b) thoracolumbar spines reveal cerebellar tonsillar herniation below the foramen magnum and holocord syringomyelia. (c) Coronal view of three-dimensional magnetic resonance myelography of the whole spine clearly demonstrates the septations in the syrinx. At the lower spinal cord, syringomyelia (arrowheads) affected predominantly on the right side.
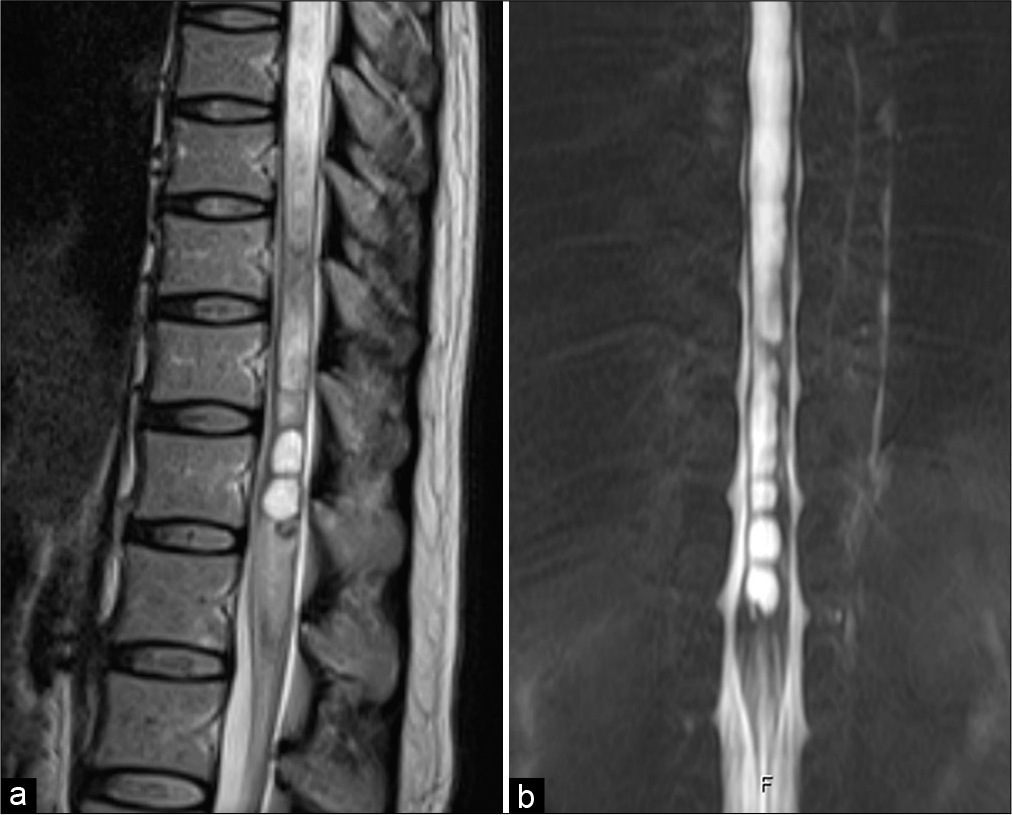
Two months later, she developed severe right-sided sciatic pain and complete right foot drop. She walked with a compensatory high steppage gait and required a cane during walking. Medical research council grading for the power strength of the right lower extremity revealed the following: iliopsoas 5/5, hip adductors 4/5, gluteus medius 4/5, quadriceps 4/5, hamstrings 4/5, tibialis anterior 0/5, extensor hallucis longus 0/5, gastrocnemius 4/5, and toes flexors 3/5. Examination of her both upper extremities and left lower extremity was normal. Follow-up full spinal MRI showed the progression of syringomyelia at the lower spinal cord and edema in the conus medullaris [
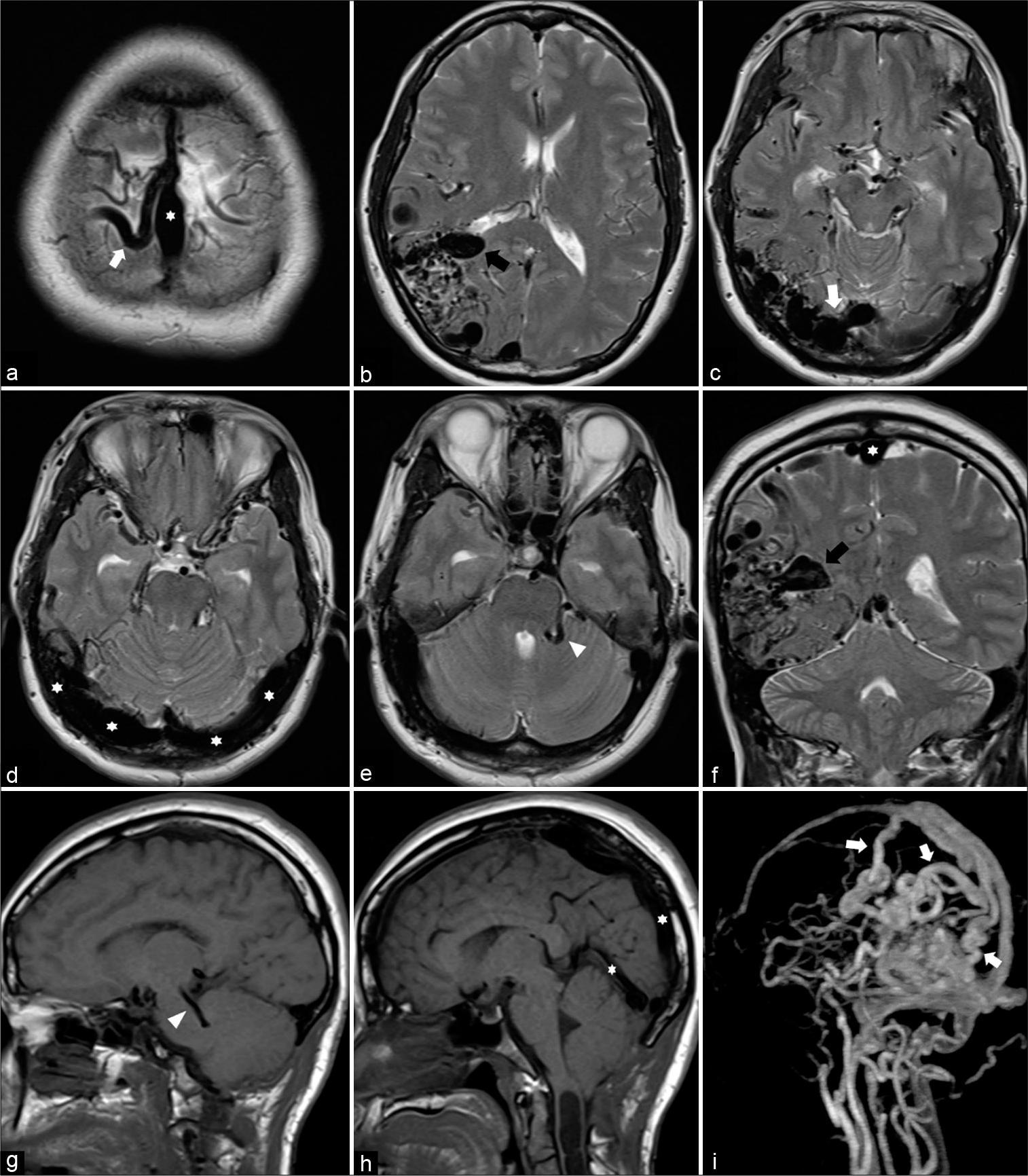
Figure 3:
(a-e) Sequential axial, (f) coronal T2-weighted, (g and h) sagittal T1-weighted magnetic resonance images, and (i) contrast-enhanced magnetic resonance angiography reveal a large brain arteriovenous malformation involving the right parietal and occipital lobes with markedly dilated cortical draining veins (arrows) and diffuse engorgement of dural venous sinuses (asterisks), including the posterior two-third portion of the superior sagittal sinus, bilateral transverse sinuses, torcula, and straight sinus. There is a large venous varix (black arrows) in the anterior portion of the nidus, probably protruded into the posterior horn of the right lateral ventricle. In addition, dilated posterior fossa veins (arrowheads) are noted.
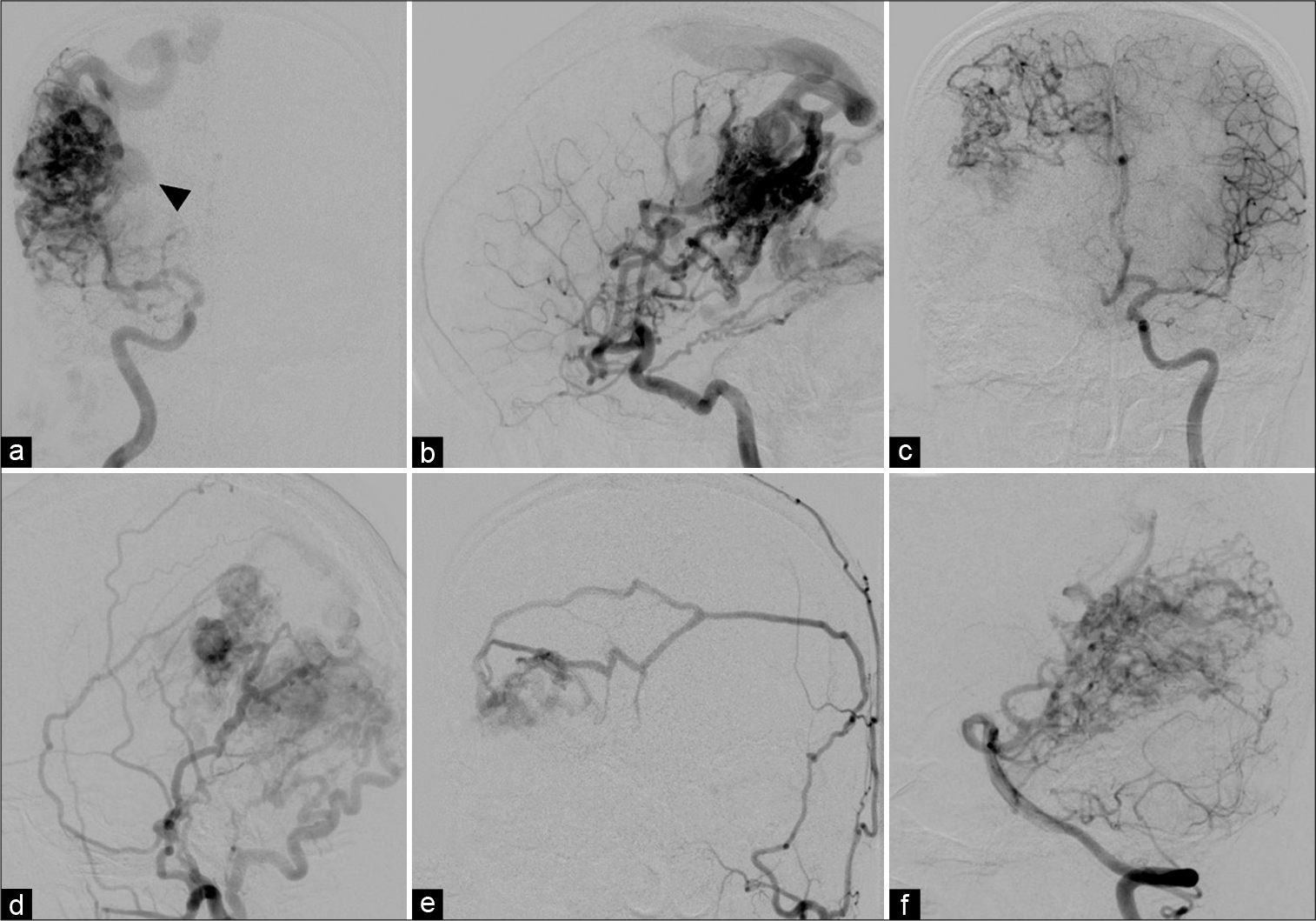
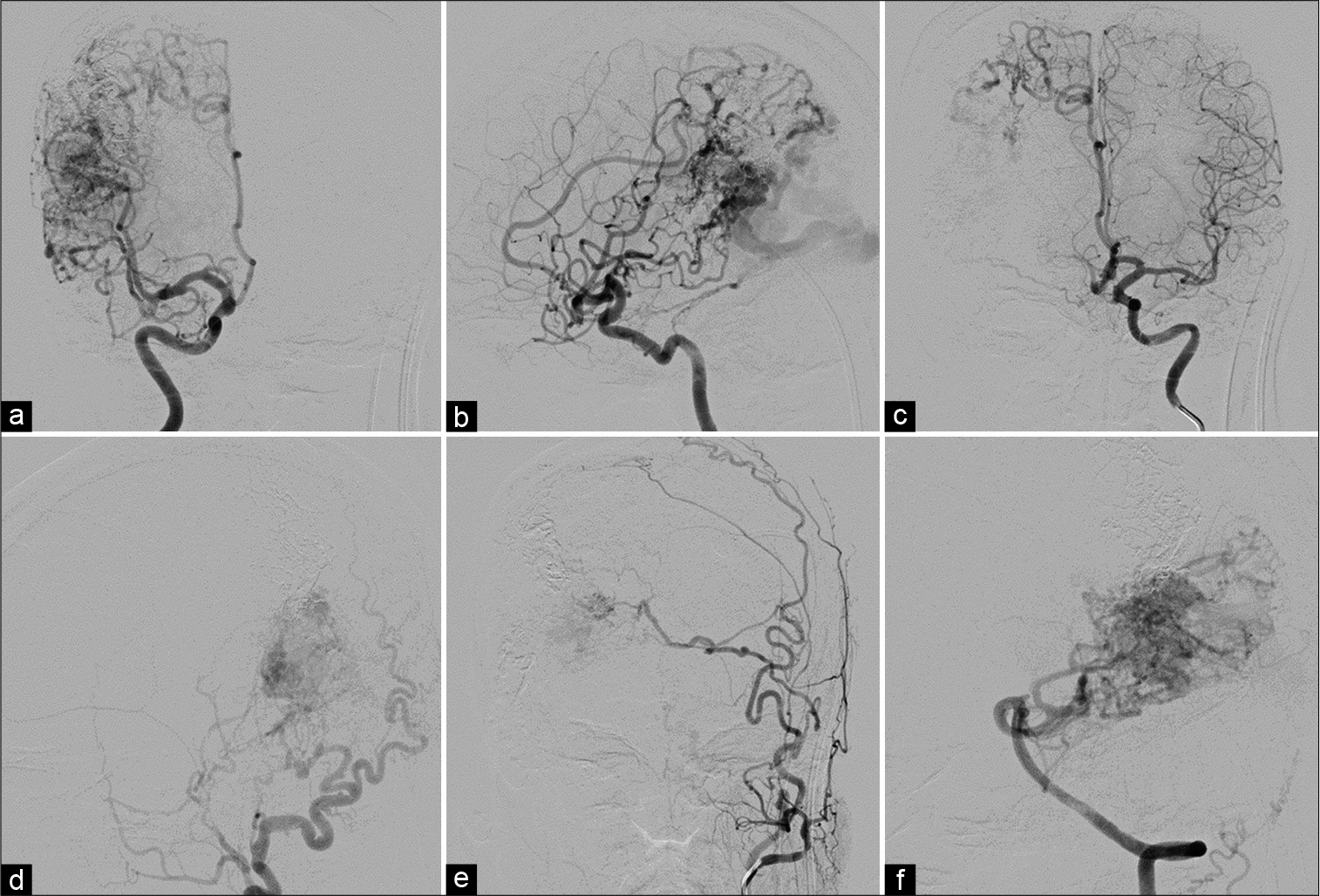
Subsequently, cerebral angiography was performed and revealed a large right parieto-occipital AVM supplied mainly by enlarged cortical branches of the right middle cerebral artery (posterior parietal and angular branches) and posterior cerebral artery (parieto-occipital and calcarine branches) with multiple dural supply. Dural supply to the AVM included the meningohypophyseal and inferolateral branches of the right cavernous ICA, bilateral middle meningeal arteries, and right occipital artery. In addition, there was indirect supply through leptomeningeal anastomosis from the cortical branches of the right anterior cerebral artery. The AVM drained predominantly toward the SSS through enlarged parietal and occipital cortical veins [
Figure 4:
(a) Anteroposterior (AP) and (b) lateral views of the right internal carotid artery (ICA), (c) AP view of the left ICA, (d) lateral view of the right external carotid artery, (e) AP view of the left external carotid artery, and (f) lateral view of the left vertebral artery injections show a large right parieto-occipital arteriovenous malformation (AVM) supplied by enlarged cortical branches of the right middle cerebral artery, and posterior cerebral artery with multiple dural supply. Dural supply to the AVM includes the meningohypophyseal and inferolateral branches of the right cavernous ICA, bilateral middle meningeal arteries, and right occipital artery. In addition, there is indirect supply through leptomeningeal anastomosis from the cortical branches of the right anterior cerebral artery. The AVM drains toward the superior sagittal sinus through enlarged cortical veins. A large venous varix (arrowhead) is noted.
Figure 5:
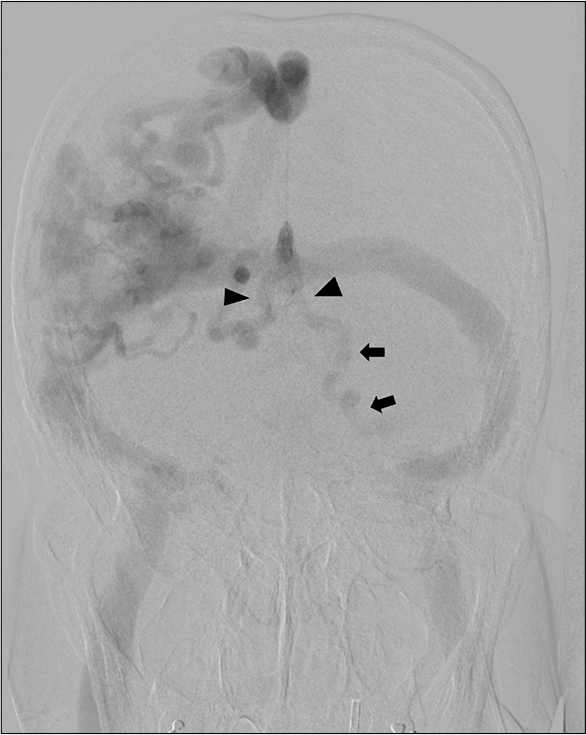
Anteroposterior view of the left vertebral artery injection illustrates retrograde venous reflux into bilateral enlarged basal veins of Rosenthal (arrowheads) and dilated vein running along left-sided brainstem (arrows) corresponding with dilated posterior fossa veins on magnetic resonance images.
The patient underwent transarterial embolization with N-butyl cyanoacrylate (NBCA) through the hypertrophic branches of the right middle cerebral artery. Superselective catheterization of the middle cerebral branches demonstrated the fistulous type of AVM [
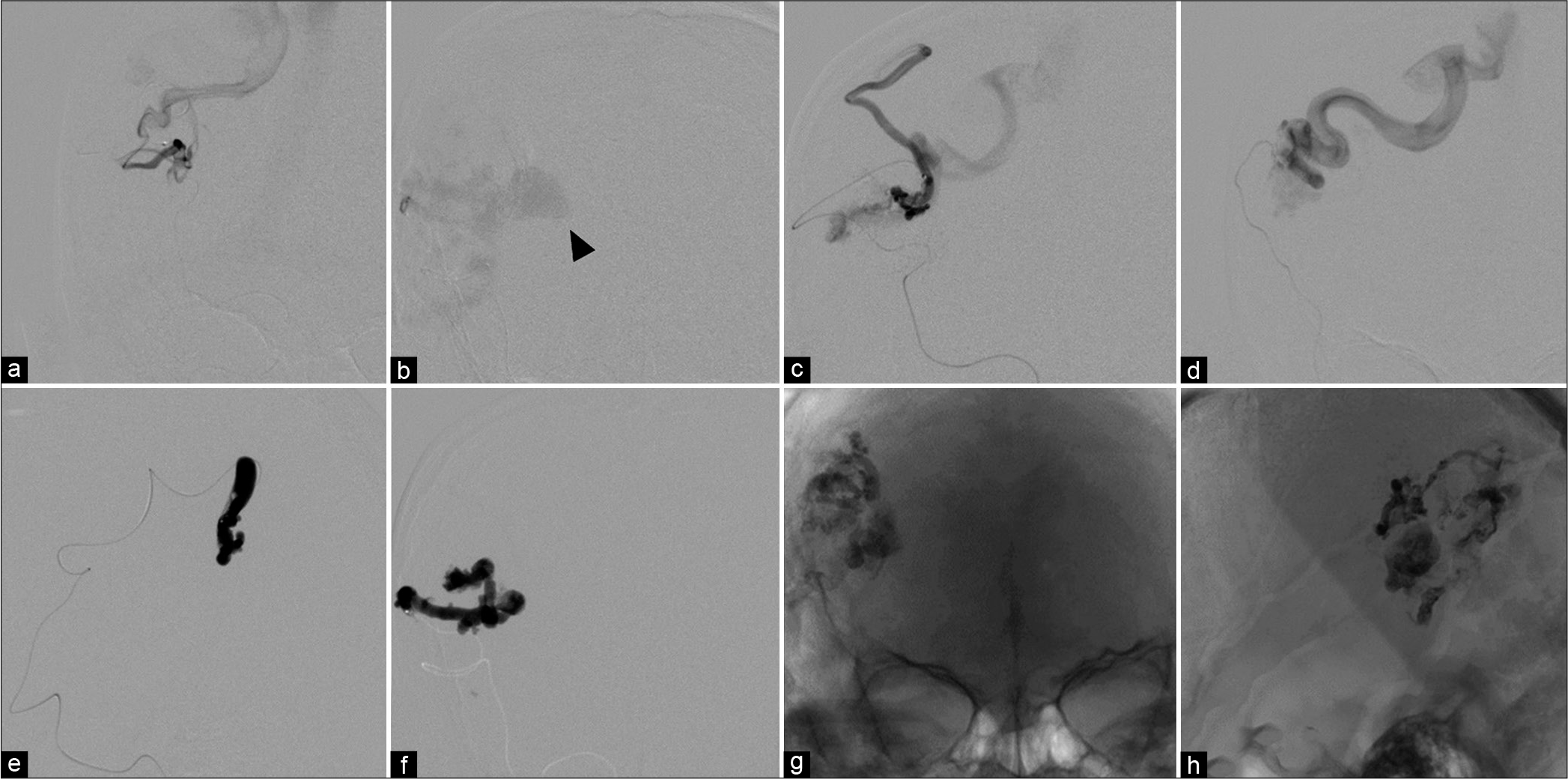
Figure 6:
(a-d) Anteroposterior views of different injections from the branches of the right middle cerebral artery through the microcatheters reveals the fistulous sites of arteriovenous malformation with markedly dilated cortical draining veins. A large venous varix (arrowhead) is noted. (e and f) During embolization with N-butyl cyanoacrylate. (g) Anteroposterior and (h) lateral views of unsubtracted image demonstrate the glue cast.
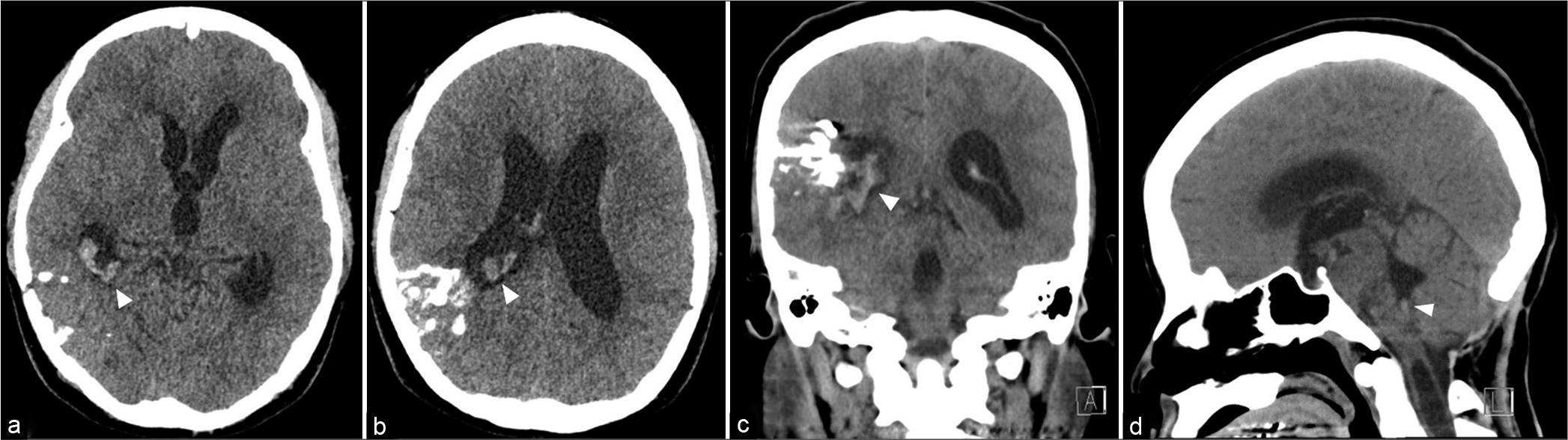
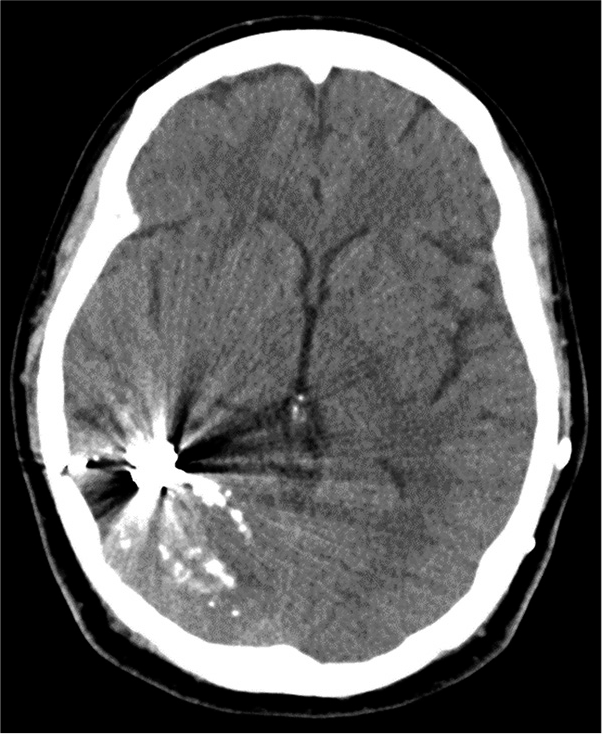
One year later, the patient developed sudden severe headache followed by alteration of conscious. She was admitted at the local hospital. Cranial computed tomography (CT) scan showed intraventricular hemorrhage in the right lateral and fourth ventricles with ventricular enlargement [
Figure 8:
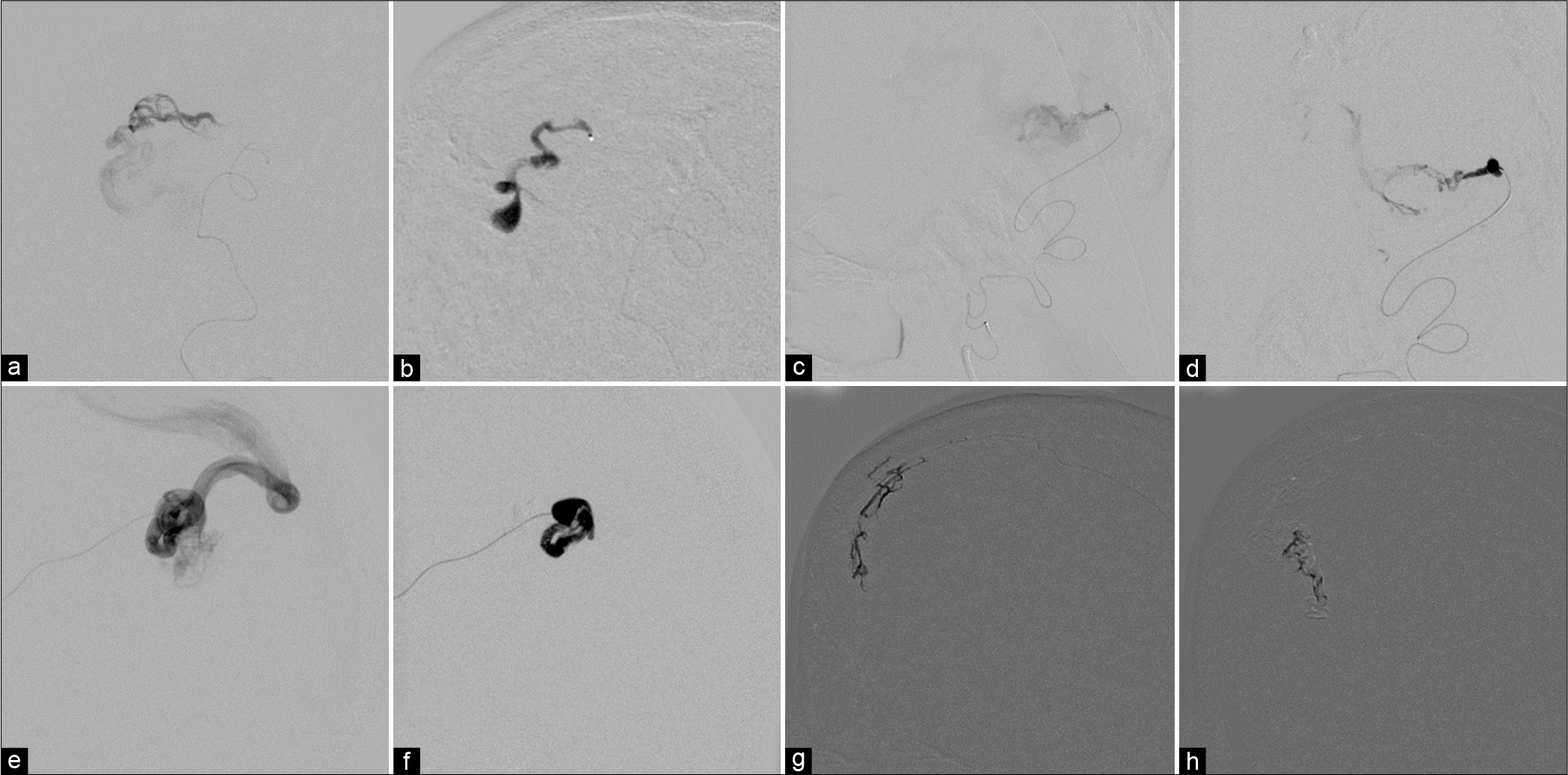
(a) Anteroposterior view of the right posterior cerebral artery, lateral view of (c) the right occipital artery, and (e) the right middle meningeal artery injections the fistulous sites of arteriovenous malformation with markedly dilated cortical draining veins. (b, d, and f) During glue injections with good penetration into the proximal draining veins. (g and h) During Onyx injections through the contralateral middle meningeal branch in anteroposterior view.
Figure 9:
Cerebral angiography obtained before radiosurgery. (a) Anteroposterior (AP), (b) lateral views of the right internal carotid artery (ICA), (c) AP view of lateral view of the left ICA, (d) lateral view of the right external carotid artery, (e) AP view of the left ECA, and (f) lateral view of the left vertebral artery injections demonstrate significant reduction in size and flow of the brain arteriovenous malformation.
DISCUSSION
Acquired CMI with syringomyelia
Acquired or secondary CMI and associated syrinx have been previously reported following lumbar puncture, lumboperitoneal shunting, and spontaneous spinal CSF leakage.[
According to the systemic review of acquired CMI and associated syrinx secondary to space-occupying lesions by Wang et al.,[
Acquired CMI associated syringomyelia caused by supratentorial mass is extremely rare.[
The pathogenesis of syringomyelia associated with acquired CMI
Based on anatomical and dynamic MRI, and intraoperative ultrasonography before, during, and after surgical decompression of the foramen magnum, Heiss et al.[
The pathogenesis of syringomyelia in patients with acquired CMI may be analogous to the development of syrinx with congenital CMI.[
Literature review of brain AVM associated with Chiari Type I malformation
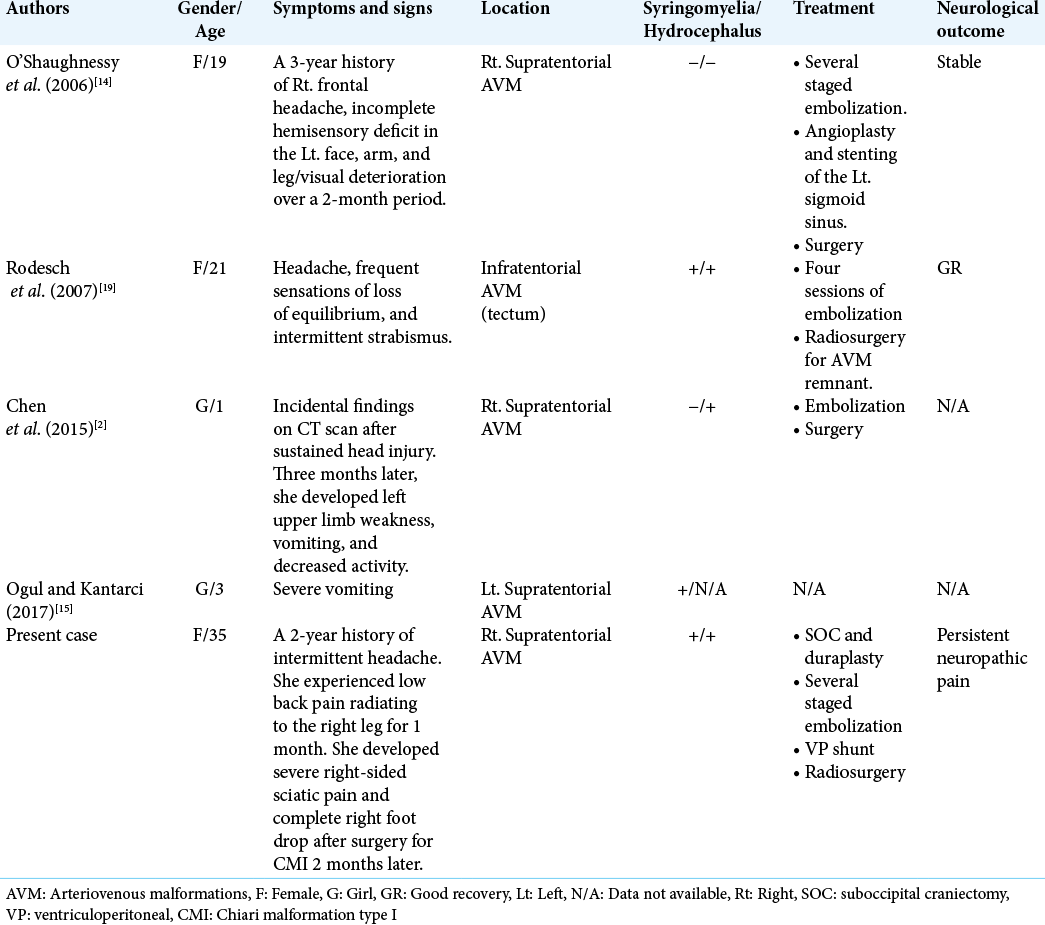
We reviewed the literature of patients with cerebral AVMs associated with CMI and found five cases, including our one case [
First, O’Shaughnessy et al.[
Regarding other type of cerebral vascular malformation associated with acquired CMI and syringomyelia, Cognard et al.[
The proposed pathophysiology of adult Chiari malformation coexisting with brain AVM in our case
A high-flow supratentorial fistulous AVM may cause hypertensive venopathy, representing by diffuse engorgement of sinuses and markedly dilated superficial cortical veins. The venous hypertension of posterior fossa was evident by the dilated posterior fossa veins. Subsequently, the venous hypertension of the posterior fossa caused pressure gradient over the craniospinal junction and resulted in CMI. Therefore, treating the CMI primarily resulted in deterioration of clinical symptoms later due to the existence of the primary cause, a large high-flow AVM. After significant reduction of the flow of the fistulous AVM, the resolution of extensive syringomyelia was seen on follow-up imaging.
CMI and associated Hydrocephalus
Hydrocephalus occurs in 3–10% of patients with CMI, particularly in children with long-standing course.[
The management of acquired CMI with syringomyelia
The goals of CMI surgery are restoring the normal flow of CSF across the foramen magnum and reduction the size of syringomyelia.[
Resection of cerebellar tonsils was not recommended due to high rate of complications. Shunting of the syrinx may worsen clinical signs and symptoms and enlarge the size of associated syrinx.
Congenital CMs should be treated with surgical decompression and duraplasty, whereas acquired CMI may be managed by treating the primary disease first.[
In the present study, CMI was misdiagnosed as congenital origin and unusual progressive enlargement of a syrinx cavity occurred following bony decompression and duraplasty. Inadequate decompression of the craniocervical junction should be considered initially. Fortunately, after detecting acquired CMI caused by supratentorial AVM, we treated the primary cause and achieved significant reduction in the syrinx cavity. The goal of endovascular treatment was aiming at reducing the flow rate of the fistulous AVM.
Holocord syringomyelia presenting as foot drop
Foot drop causes predominantly from the peripheral nervous system. CMI and associated holocord syrinx are an extremely rare central cause of foot drop and <10 cases are found in the literature, usually occurring in childhood or adolescence. Most patients complained abrupt onset or rapidly progressive foot drop. Early diagnosis of CMI and holocord syrinx is pivotal and prompt surgical decompression may lead to an excellent prognosis.[
Syringomyelia and neuropathic pain
Persistent central neuropathic pain may cause by intramedullary lesion such as intramedullary hemorrhage.[
CONCLUSION
We reported the fifth case of acquired CMI with associated syringomyelia secondary to brain AVM. The development of acquired CMI and holocord syringomyelia may cause by intracranial venous hypertension resulting from supratentorial AVM-induced venopathy. Complication of intraventricular hemorrhage from the AVM may precipitate the occurrence of hydrocephalus requiring CSF diversion. Endovascular treatment of brain AVM achieved successful management of holocord syringomyelia, worsening after suboccipital craniectomy and duraplasty. A thorough understanding of the pathogenesis and natural history of the coexistence of CMI with syringomyelia and intracranial AVM are imperative in guiding the proper management of this entity. The need for cranial imaging in initial evaluation of cases with adult Chiari malformation is important.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Atkinson JL, Weinshenker BG, Miller GM, Piepgras DG, Mokri B. Acquired Chiari I malformation secondary to spontaneous spinal cerebrospinal fluid leakage and chronic intracranial hypotension syndrome in seven cases. J Neurosurg. 1998. 88: 237-42
2. Chen KW, Kuo MF, Lee CW, Tu YK. Acquired Chiari malformation Type I associated with a supratentorial fistulous arteriovenous malformation: A case report. Childs Nerv Syst. 2015. 31: 499-501
3. Cognard C, Casasco A, Toevi M, Houdart E, Chiras J, Merland JJ. Dural arteriovenous fistulas as a cause of intracranial hypertension due to impairment of cranial venous outflow. J Neurol Neurosurg Psychiatry. 1998. 65: 308-16
4. Fernández AA, Guerrero AI, Martínez MI, Vázquez ME, Fernández JB, Chesa i Octavio E. Malformations of the craniocervical junction (Chiari Type I and syringomyelia: Classification, diagnosis and treatment). BMC Musculoskelet Disord. 2009. 10: S1
5. Heiss JD, Patronas N, DeVroom HL, Shawker T, Ennis R, Kammerer W. Elucidating the pathophysiology of syringomyelia. J Neurosurg. 1999. 91: 553-62
6. Holly LT, Batzdorf U. Chiari malformation and syringomyelia. J Neurosurg Spine. 2019. 31: 619-28
7. Iampreechakul P, Lertbutsayanukul P, Siriwimonmas S, Jittapiromsak P, Tantivatana J, Niruthisard S. Persistent central neuropathic pain caused by intramedullary hemorrhage from spinal dural arteriovenous fistula: A case report and literature review. Austin J Anesth Analg. 2019. 7: 1076
8. Jayamanne C, Fernando L, Mettananda S. Chiari malformation Type 1 presenting as unilateral progressive foot drop: A case report and review of literature. BMC Pediatr. 2018. 18: 34
9. Kosary IZ, Braham J, Shaked I, Tadmor R. Cervical syringomyelia associated with occipital meningioma. Neurology. 1969. 19: 1127-30
10. Lee M, Rezai AR, Wisoff JH. Acquired Chiari-I malformation and hydromyelia secondary to a giant craniopharyngioma. Pediatr Neurosurg. 1995. 22: 251-4
11. Martínez-Lage JF, Almagro MJ, Ros de San Pedro J, RuizEspejo A, Felipe-Murcia M. Regression of syringomyelia and tonsillar herniation after posterior fossa arachnoid cyst excision. Case report and literature review. Neurocirugia (Astur). 2007. 18: 227-31
12. McMillan HJ, Sell E, Nzau M, Ventureyra EC. Chiari 1 malformation and holocord syringomyelia presenting as abrupt onset foot drop. Childs Nerv Syst. 2011. 27: 183-6
13. Morioka T, Shono T, Nishio S, Yoshida K, Hasuo K, Fukui M. Acquired Chiari I malformation and syringomyelia associated with bilateral chronic subdural hematoma. Case report. J Neurosurg. 1995. 83: 556-8
14. O’Shaughnessy BA, Bendok BR, Parkinson RJ, Shaibani A, Walker MT, Shakir E. Acquired Chiari malformation Type I associated with a supratentorial arteriovenous malformation. Case report and review of the literature. J Neurosurg. 2006. 104: 28-32
15. Ogul H, Kantarci M. Unusual Association: Cerebral Arteriovenous Malformation and Chiari Type I Malformation. J Craniofac Surg. 2017. 28: e376-7
16. Onesti ST, Ashkenazi E, Miller AM, Michelsen WJ. Resolution of acquired tonsillar herniation after resection of supratentorial meningioma. Case illustration. J Neurosurg. 1997. 86: 572
17. Panda AK, Kaur M. Rapidly progressive foot drop: An uncommon and underappreciated cause of Chiari I malformation and holocord syrinx. BMJ Case Rep. 2013. 2013: bcr2013009644
18. Peleggi AF, Lovely TJ. Treatment of delayed Chiari malformation and syringomyelia after lumboperitoneal shunt placement: Case report and treatment recommendations. Surg Neurol Int. 2012. 3: 101
19. Rodesch G, Otto B, Mouchamps M, Born J. Reversible tonsillar prolapse and syringomyelia after embolization of a tectal arteriovenous malformation. Case report and review of the literature. J Neurosurg. 2007. 107: 412-5
20. Ryba B, Lewis CS, Diaz-Aguilar LD, Pham M. Surgical decompression for holocord syringomyelia from chiari malformation: Case report and systematic review. Interdiscip Neurosurg. 2021. 23: 100907
21. Sathi S, Stieg PE. “Acquired” Chiari I malformation after multiple lumbar punctures: Case report. Neurosurgery. 1993. 32: 306-9
22. Seki T, Hamauchi S,Yamazaki M, Hida K, Yano S, Houkin K. Investigation of the neuropathic pain caused by syringomyelia associated with Chiari I malformation. Asian Spine J. 2019. 13: 648-53
23. Sheehan JM, Jane JA Sr. Resolution of tonsillar herniation and syringomyelia after supratentorial tumor resection: Case report and review of the literature. Neurosurgery. 2000. 47: 233-5
24. Thotakura AK, Marabathina NR. Acquired Chiari I malformation with syringomyelia secondary to colloid cyst with hydrocephalus-case report and review of literature. World Neurosurg. 2017. 108: 995.e1-4
25. Wang J, Alotaibi NM, Samuel N, Ibrahim GM, Fallah A, Cusimano MD. Acquired chiari malformation and syringomyelia secondary to space-occupying lesions: A systematic review. World Neurosurg. 2017. 98: 800-8.e2
26. Zhao JL, Li MH, Wang CL, Meng W. A systematic review of Chiari I malformation: Techniques and outcomes. World Neurosurg. 2016. 88: 7-14