- Department of Neurosurgery, Stanford University School of Medicine, Stanford, United States.
Correspondence Address:
Michael Zhang Department of Neurosurgery, Stanford University School of Medicine, Stanford, United States.
DOI:10.25259/SNI_671_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Michael Zhang1, Parastou Fatemi1, Jamshid Ghajar1. Acquired dural arteriovenous fistula after subdural evacuation port system placement: A case report. 18-Nov-2022;13:540
How to cite this URL: Michael Zhang1, Parastou Fatemi1, Jamshid Ghajar1. Acquired dural arteriovenous fistula after subdural evacuation port system placement: A case report. 18-Nov-2022;13:540. Available from: https://surgicalneurologyint.com/surgicalint-articles/12006/
Abstract
Background: The subdural evacuation port system (SEPS) is a rapid, bedside, and less invasive option for subdural hemorrhage management. Proper procedure planning and understanding of the relevant vascular anatomy is important for minimizing complications and future procedures.
Case Description: We describe a case where following placement of a SEPS, there was immediate development of a new dural arteriovenous fistula (dAVF) between the middle meningeal artery (MMA) and middle meningeal vein. Angiography confirmed site of shunting to be at the proximity of the twist drill hole placement. Subsequent MMA embolization was performed and follow-up MRI confirmed resolution of the dAVF.
Conclusion: SEPS-associated dAVF is an underreported complication with potential long-term consequences. This case describes the complication and advocates avoiding SEPS anterior to the coronal suture.
Keywords: Case report, Dural arteriovenous fistula, Middle meningeal, Subdural hemorrhage
INTRODUCTION
Dural arteriovenous fistula (dAVF) is an extracranial arterial blood flow shunt within the leaflets of the dura mater.[
Here, we present the first reporting of a dAVF following subdural evacuation port system (SEPS) placement for the evacuation of a subdural hematoma (SDH). Patient consent was obtained. The case provides insight into the possible development timeline of an acquired dAVF. Moreover, this case report offers anatomic guidance on the surgical planning of SEPS for hematoma management and relevant postoperative treatment for postsurgical dAVF.
CLINICAL DESCRIPTION
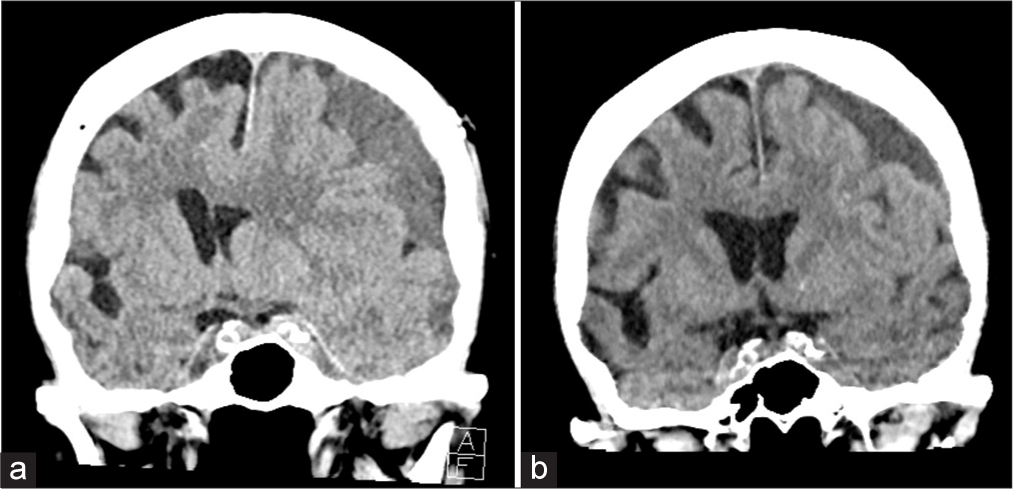
A 93-year-old female on apixaban for atrial fibrillation presented after a ground level fall with difficulty speaking and an otherwise intact neurological examination. Her head computed tomography (CT) was trended until stable and identified a 16 mm SDH with 9 mm midline shift (MLS; [
Figure 1:
Coronal computed tomography (CT) head imaging of the patient at (a) initial presentation and (b) 2-week follow-up, after discharge from her subdural evacuation and middle meningeal embolization. Initial presentation demonstrated a 15 mm midline shift. Final follow-up CT described a 3 mm midline shift.
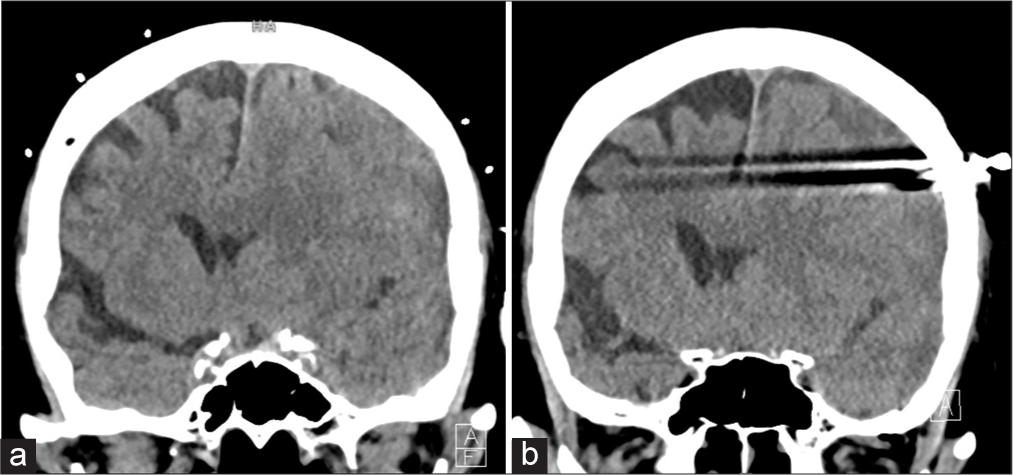
She had a frontal SEPS drainage placement and a postprocedural CT head showed interval decrease in the SDH to 1.7 cm with an 11 mm MLS [
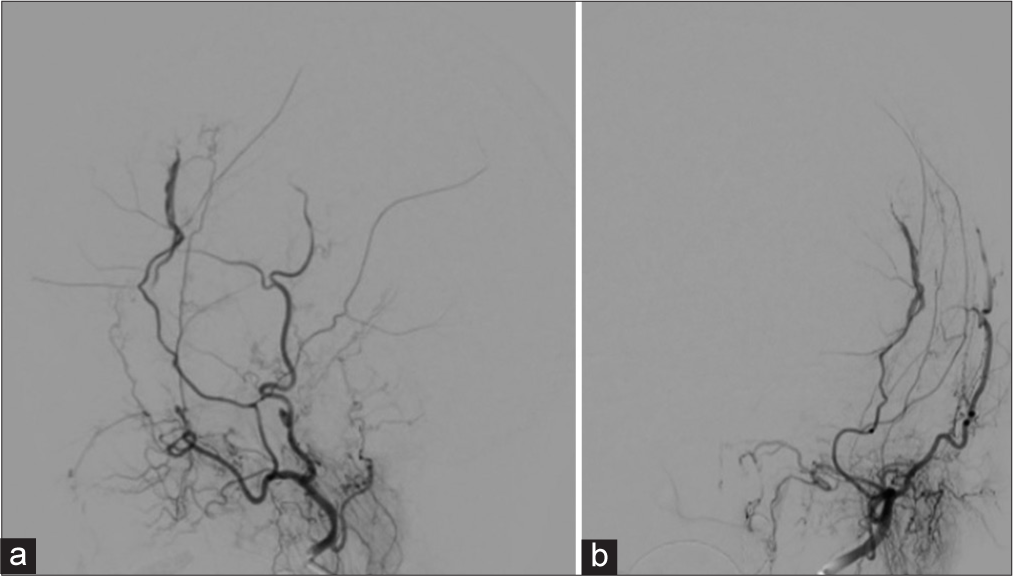
Figure 3:
Dual subtraction angiography of the left external carotid from (a) lateral and (b) anterior-posterior views following SEPS drainage demonstrating the anterior left middle meningeal artery supplying a postsurgical arteriovenous fistula with drainage into the left middle meningeal vein, without cortical venous drainage.
A week later, she was at her normal neurological baseline. After additional monitoring, she was ultimately discharged with recommendations to hold anticoagulation for 6 weeks. At 2-week follow-up, a repeat CT head scan identified further reduction in the SDH, measuring 1.5 cm with a 3 mm MLS.
DISCUSSION
Iatrogenic dural arteriovenous fistula have been associated with surgical interventions, but primarily intracranial procedures.[
Similar traumatic MMA-MMV dAVF have been described and have radiographically been characterized to have a “tram-track” presentation.[
Two case reports of ventriculostomy associated dAVF have been described; however, both were associated with aneurysmal subarachnoid hemorrhage.[
This case also demonstrates the possible significance of SEPS site selection. The coronal suture is an important landmark as the MMA trajectory runs posterior to it.[
CONCLUSION
Dural injury with a twist drill can lead to formation of a dural arteriovenous fistula within the same day of the associated dural trauma. Surgical planning should incorporate the underlying arterial and venous anatomy to reduce the need for subsequent follow-up and treatment.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Ahn JY, Kim OJ, Joo YJ, Joo JY. Dural arteriovenous malformation occurring after craniotomy for pial arteriovenous malformation. J Clin Neurosci. 2003. 10: 134-6
2. Aminoff MJ. Vascular anomalies in the intracranial dura mater. Brain. 1973. 96: 601-12
3. Field M, Branstetter BF, Levy E, Yonas H, Jungreis CA. Dural arteriovenous fistula after ventriculostomy. Case illustration. J Neurosurg. 2002. 97: 227
4. Hwang SC, Im SB, Kim BT, Shin WH. Safe entry point for twist-drill craniostomy of a chronic subdural hematoma. J Neurosurg. 2009. 110: 1265-70
5. Igase K, Oka Y, Kumon Y, Zenke K, Iwata S, Sakaki S. A case of dural AVM detected after STA-MCA anastomosis. No Shinkei Geka. 1996. 24: 81-5
6. Kim SW, Chae KS, Shim JH, Rho SJ, Choi HK, Park HS. Iatrogenic dural arteriovenous fistula after superficial temporal artery to middle cerebral artery anastomosis: A case report. Korean J Neurotrauma. 2015. 11: 151-3
7. Luther EM, Chagani F, King H, Starke R. Rupture of a de novo dural AV fistula following adult cerebral AVM resection. BMJ Case Rep. 2021. 14: e246758
8. Miller TR, Gandhi D. Intracranial dural arteriovenous fistulae: Clinical presentation and management strategies. Stroke. 2015. 46: 2017-25
9. Miura N, Kadota K, Ogawa N, Shinohara T, Shimizu T. A case of arteriovenous communication between external and internal carotid arteries and the sinus after removal of the meningioma (author’s transl). No Shinkei Geka. 1975. 3: 265-9
10. Nabors MW, Azzam CJ, Albanna FJ, Gulya AJ, Davis DO, Kobrine AI. Delayed postoperative dural arteriovenous malformations, Report of two cases. J Neurosurg. 1987. 66: 768-72
11. Pappas CT, Zabramski JM, Shetter AG. Iatrogenic arteriovenous fistula presenting as a recurrent subdural hematoma. Case report. J Neurosurg. 1992. 76: 134-6
12. Satomi J, van Dijk JM, Terbrugge KG, Willinsky RA, Wallace MC. Benign cranial dural arteriovenous fistulas: Outcome of conservative management based on the natural history of the lesion. J Neurosurg. 2002. 97: 767-70
13. Shotar E, Mathon B, Meyblum L, Lenck S, Premat K, Degos V, editors. Letter to the editor regarding “immediate development of dural arteriovenous fistula after middle meningeal artery embolization: First angiographic demonstration”. World Neurosurg. 2019. 131: 295-6
14. Tabibian BE, Liptrap E, Jones J. Incidentally discovered dural arteriovenous fistula during middle meningeal artery embolization for the treatment of chronic subdural hematoma. Surg Neurol Int. 2021. 12: 438
15. Vadivelu S, Xin X, Loven T, Restrepo G, Chalif DJ, Setton A. Iatrogenic dural arteriovenous fistula and aneurysmal subarachnoid hemorrhage. Neurosurg Focus. 2012. 32: E1
16. Watanabe A, Takahara Y, Ibuchi Y, Mizukami K. Two cases of dural arteriovenous malformation occurring after intracranial surgery. Neuroradiology. 1984. 26: 375-80
17. Yassari R, Jahromi B, Macdonald R. Dural arteriovenous fistula after craniotomy for pilocytic astrocytoma in a patient with protein S deficiency. Surg Neurol. 2002. 58: 59-64 discussion 64