- Department of Neurosurgery, School of Medicine, Georgetown University, Washington, DC, USA.
- Department of Pharmacy, Massachusetts General Hospital, Boston, MA, USA.
- Department of Pathology, Massachusetts General Hospital, Boston, MA, USA.
- Department of Neurosurgery, Massachusetts General Hospital, Boston, MA, USA.
Correspondence Address:
Nicholas Dietz
Department of Neurosurgery, Massachusetts General Hospital, Boston, MA, USA.
DOI:10.25259/SNI_225_2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nicholas Dietz, Megan Barra, Mingjuan Zhang, Marcus Zacharaiah, Jean-Valery Coumans. Acute myeloid leukemia with central nervous system extension and subdural seeding of vancomycin-resistant Enterococcus faecium after bilateral subdural hematomas treated with subdural daptomycin administration. 10-Sep-2019;10:171
How to cite this URL: Nicholas Dietz, Megan Barra, Mingjuan Zhang, Marcus Zacharaiah, Jean-Valery Coumans. Acute myeloid leukemia with central nervous system extension and subdural seeding of vancomycin-resistant Enterococcus faecium after bilateral subdural hematomas treated with subdural daptomycin administration. 10-Sep-2019;10:171. Available from: http://surgicalneurologyint.com/surgicalint-articles/9617/
Abstract
Background: We present a rare case of comorbid relapsed acute myeloid leukemia (AML) with the involvement of the central nervous system (CNS) and subdural seeding of vancomycin-resistant Enterococcus faecium (VRE). The safety profile, treatment approach with pharmacokinetic considerations, and evaluation of success for bilateral subdural administration of daptomycin after subdural hematoma (SDH) are assessed.
Case Description: A 45-year-old male with a history of AML who underwent chemotherapy (induction with 7 + 3) was admitted to oncology with relapsed AML confirmed by bone marrow biopsy, complicated by neutropenic fever and VRE bacteremia. After acute neurological changes with image confirmation of mixed- density bilateral SDHs secondary to thrombocytopenia, the patient was admitted to the neurosurgery unit and underwent bilateral burr hole craniotomies for subdural evacuation with the placement of the left and right subdural drains. Culture of the subdural specimen confirmed VRE seeding of the subdural space. The patient received the first dose of daptomycin into the bilateral subdural spaces 2 days after evacuation and was noted to have acute improvement on neurological examination, followed by a second administration to the left subdural space 5 days after evacuation with bilateral drains pulled thereafter.
Conclusion: In this patient, the complication of relapsed AML may have contributed to the rare extension of VRE into the CNS space. Screening for patients at risk of AML with CNS involvement and addressing coagulopathy and risk of infection may help mitigate morbidity. Bilateral administration of subdural daptomycin bolus into the subdural space was tolerated and possibly contributed to the patient’s neurological improvement during an extended hospital course.
Keywords: Acute myeloid leukemia with central nervous system involvement, Acute myeloid leukemia, Daptomycin, Subdural administration, Subdural hematoma
INTRODUCTION
Incidence of central nervous system (CNS) involvement by acute myeloid leukemia (AML) in adult patients is reported to be 0.6%–2%[
Enterococcal meningitis comprises only 0.3%–4% of bacterial meningitis cases.[
Given the rapid bactericidal efficacy of daptomycin against multidrug-resistant pathogens, efforts have been made to deliver the antibiotic through alternative methods to treat intracranial infections.[
CASE REPORT
A 45-year-old male presented with relapsed AML with CNS involvement, complicated by neutropenic fever and sepsis with VRE bacteremia and subsequently developed bilateral subdural hematomas (SDHs) with seeding of VRE into the subdural spaces bilaterally. The patient was initially diagnosed with AML (FLT3/NPM1 wild type, trisomy 7, 7q and 22q deletions, and TET2 and UT2AF1 mutations) approximately 2 months before admission and was started on 7 + 3 therapy (cytarabine and daunorubicin). On outpatient oncology follow-up, he was found to have a right-sided facial droop secondary to Bell’s palsy and admitted to the emergency department, where a head computed tomography (CT) was negative. The patient was subsequently admitted to an outside hospital for nausea and vomiting with a lactate dehydrogenase of 955 and white blood cell count of 78,000 with 35% blasts, for which he was initiated on allopurinol and hydroxyurea and transferred to our institution’s hospital for further medical workup and bone marrow transplant evaluation.
On admission to our institution’s hospital, the patient was afebrile and hemodynamically stable. The patient was alert and oriented though unable to recall the details of previous hospital stays. He endorsed a 20 lbs weight loss over the past 3 months and intermittent 10/10 occipital throbbing headaches. The patient underwent a bone marrow biopsy, which showed relapsed AML, for which he completed high-dose cytarabine and mitoxantrone (HAM) reinduction. Due to new-onset Bell’s palsy, CSF specimens were obtained; pathology and flow cytometry reports were consistent with AML with CNS involvement,
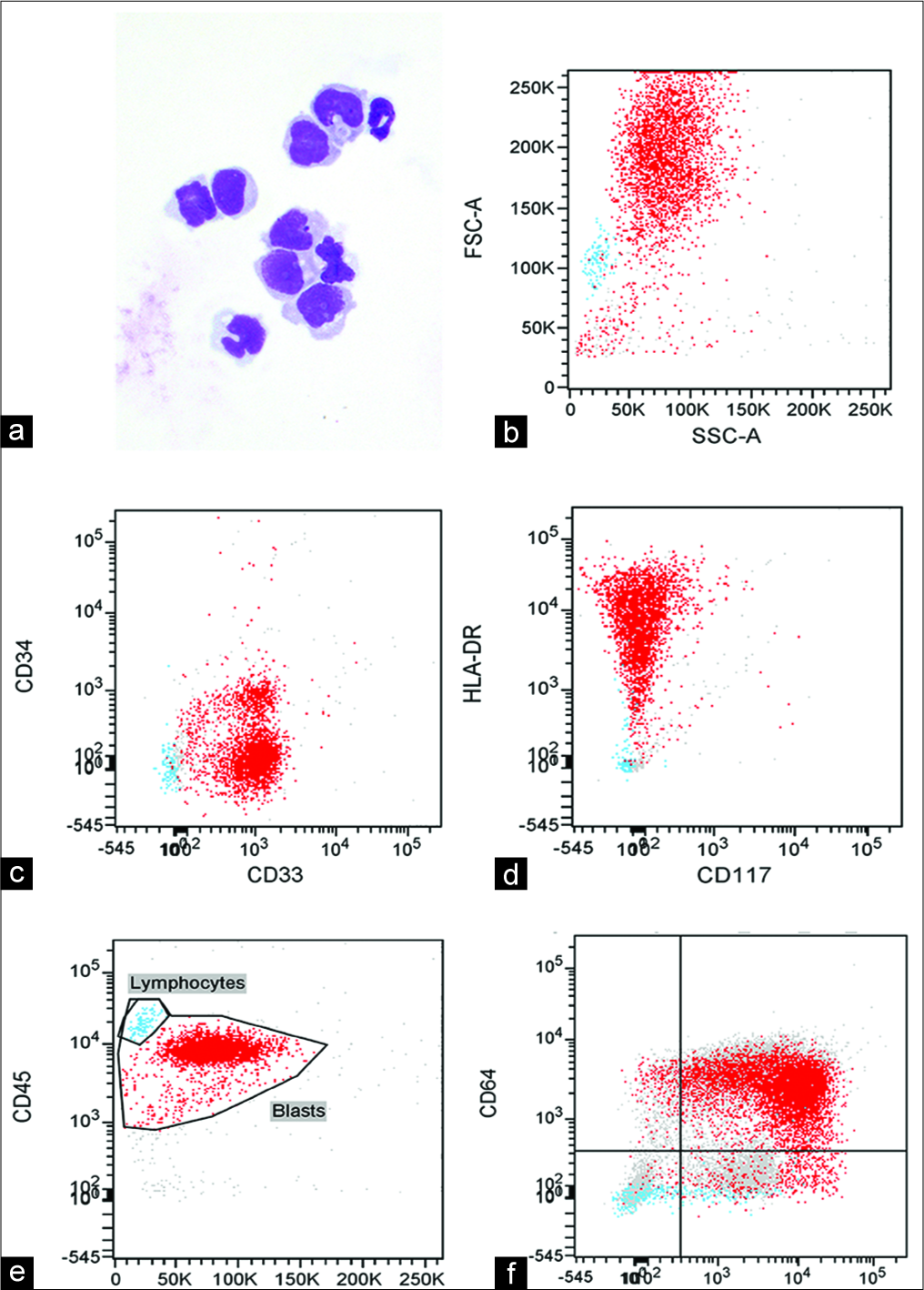
Figure 1:
(a) Cerebrospinal fluid (CSF) cytology of Giemsa- and Papanicolaou-stained cytospin preparations showing monoblasts/ promonocytes with round-to-folded nuclei, fine chromatin, and prominent nucleoli consistent for acute myeloid leukemia (AML) with monoblastic/monocytic morphology (Diff-Quik, ×600). (b-e) Flow cytometry of the CSF using FACSDiva software using CD45 versus side scatter gating showing a large myeloid blast population (91% of all cells). The blasts were CD45dim, CD33+, CD34–, CD117–, and HLA-DRbright, consistent with central nervous system involvement by the patient’s AML with monocytic differentiation. (f) Flow cytometry of bone marrow showing expression of monocytic markers (CD64 and CD11c) in the blast population.
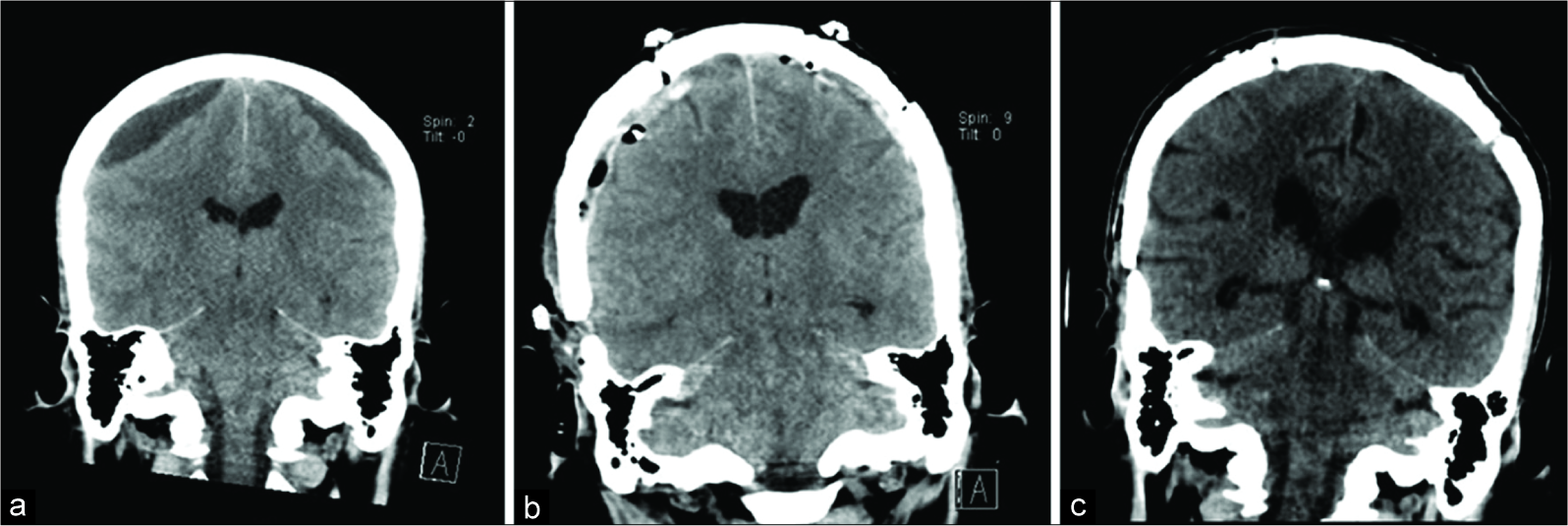
Thirty-one days after admission, the patient was intubated for inability to protect his airway after decompensation with increased emesis and somnolence. He was found to have acute bilateral mixed-density SDHs measuring 8 mm on the left and 5 mm on the right in the setting of profound thrombocytopenia (platelet count = 7 K/µL),
Daptomycin 2.5 mg in sodium chloride 0.9% 2 ml solution with 1–2 ml saline flush was administered to the left subdural drain 72 h after the initial bolus dose (POD #5). Subdural daptomycin administration was limited to the left subdural drain in a setting of a nonfunctioning right subdural drain requiring removal. The patient remained on IV daptomycin, linezolid, and imipenem/cilastatin for broad-spectrum antibiotic coverage during this time and after subdural administration of daptomycin. The subdural drains were pulled after the second administration of daptomycin on POD #5 and the patient was transferred out of the ICU with planned bone marrow biopsy and reinduction-HAM chemotherapy.
The patient’s hospital course was complicated by the observation of delta discharges over the left centroparietal and posterior regions consistent with ictal to interictal spectrum with the right upper limb and hand twitching indicating Epilepsia partialis continua on POD #11 through POD #19. The right-sided myoclonic jerk was noted to increase on deliberate movement of the right upper extremity, i.e., holding his arms outstretched in front of him and not noted at rest. Due to the acute onset, focal seizure secondary to cortical irritation from blood products or subdural daptomycin was suspected versus hemispasm secondary to Bell’s palsy. Additional findings included generalized polymorphic theta slowing with prominent left temporal theta slowing. The patient was without clinical improvement after treatment with levetiracetam 1500 mg twice daily and phenytoin therapy. He was transitioned to levetiracetam 1500 mg twice daily and lacosamide 200 mg twice daily on hospital discharge.
DISCUSSION
We report a unique presentation of CNS involvement by AML with monocytic differentiation, development of bilateral hematomas, and subdural VRE infection in the same patient. Traditional risk factors for CNS involvement of AML include elevated lactate dehydrogenase, hyperleukocytosis, CD56+ blast cells, and a prominent monocytic component.[
Furthermore, evidence suggests that CNS involvement in AML increases the propensity for subdural hemorrhage and other intracranial bleeds.[
The previous reports have demonstrated an acceptable safety profile of subdural daptomycin for the treatment of VRE in the subdural space.[
Analysis of peak and trough levels with follow-up culture of samples was attempted and would have improved our ability to evaluate the efficacy of daptomycin treatment for subdural seeding of VRE. In addition, daptomycin accumulation and discordance between the left and right spaces would assist in the evaluation of therapy and provide metrics for future administrations in the subdural microenvironments. Samples obtained from the subdural drain before and after subdural administrations may inform the degree to which IV antibiotics contributed to improvement or confounded subdural administrations. It may have been more appropriate to place an Ommaya reservoir for the consistent and reliable long-term administration and monitoring of subdural antibiotics. As Ommaya placement has been previously demonstrated to be successful for VRE ventriculitis,[
The complication of relapsed AML with monocytic differentiation may have contributed to the rare extension of VRE into the CNS space in our patient. Screening for patients at risk of AML with CNS involvement and addressing coagulopathy and risk of infection may help mitigate morbidity in future cases. Bilateral administration of subdural daptomycin was tolerated and possibly contributed to the patient’s neurological improvement during his extended hospital course.
CONCLUSION
Subdural administration of daptomycin may be an appropriate treatment option for clinicians faced with managing this rare CNS infection in this complicated clinical setting.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alakel N, Stölzel F, Mohr B, Kramer M, Oelschlägel U, Röllig C. Symptomatic central nervous system involvement in adult patients with acute myeloid leukemia. Cancer Manag Res. 2017. 9: 97-102
2. Castagnola C, Nozza A, Corso A, Bernasconi C. The value of combination therapy in adult acute myeloid leukemia with central nervous system involvement. Haematologica. 1997. 82: 577-80
3. Chuang YC, Lin HY, Chen PY, Lin CY, Wang JT, Chang SC. Daptomycin versus linezolid for the treatment of vancomycin-resistant enterococcal bacteraemia: Implications of daptomycin dose. Clin Microbiol Infect. 2016. 22: 890-7
4. Denetclaw TH, Suehiro I, Wang PK, Tolliver GL. Successful treatment of ventriculostomy-associated meningitis caused by multidrug resistant coagulase-negative Staphylococcus epidermidis using low-volume intrathecal daptomycin and loading strategy. Ann Pharmacother. 2014. 48: 1376-9
5. Elvy J, Porter D, Brown E. Treatment of external ventricular drain-associated ventriculitis caused by enterococcus faecalis with intraventricular daptomycin. J Antimicrob Chemother. 2008. 61: 461-2
6. Gonzalez-Ruiz A, Seaton RA, Hamed K. Daptomycin: An evidence-based review of its role in the treatment of gram-positive infections. Infect Drug Resist. 2016. 9: 47-58
7. Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH. Refinement of cytogenetic classification in acute myeloid leukemia: Determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom medical research council trials. Blood. 2010. 116: 354-65
8. Humphries RM, Pollett S, Sakoulas G. A current perspective on daptomycin for the clinical microbiologist. Clin Microbiol Rev. 2013. 26: 759-80
9. Jaspan HB, Brothers AW, Campbell AJ, McGuire JK, Browd SR, Manley TJ. Multidrug-resistant Enterococcus faecium meningitis in a toddler: Characterization of the organism and successful treatment with intraventricular daptomycin and intravenous tigecycline. Pediatr Infect Dis J. 2010. 29: 379-81
10. Jourdan E, Dombret H, Glaisner S, Micléa JM, Castaigne S, Degos L. Unexpected high incidence of intracranial subdural haematoma during intensive chemotherapy for acute myeloid leukaemia with a monoblastic component. Br J Haematol. 1995. 89: 527-30
11. Kahler KH.editors. Successful use of intrathecal daptomycin to treat meningitis due to vancomycin-resistant Enterococcus faecium. Infect Dis Clin Pract. 2012. p. 1-3
12. Lich BF, Conner AK, Burks JD, Glenn CA, Sughrue ME. Intrathecal/Intraventricular linezolid in multidrug-resistant Enterococcus faecalis ventriculitis. J Neurol Surg Rep. 2016. 77: e160-1
13. Miller ES, Dias PS, Uttley D. Management of subdural empyema: A series of 24 cases. J Neurol Neurosurg Psychiatry. 1987. 50: 1415-8
14. Mueller SW, Kiser TH, Anderson TA, Neumann RT. Intraventricular daptomycin and intravenous linezolid for the treatment of external ventricular-drain-associated ventriculitis due to vancomycin-resistant Enterococcus faecium. Ann Pharmacother. 2012. 46: e35-
15. Nau R, Sörgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev. 2010. 23: 858-83
16. O’Driscoll T, Crank CW. Vancomycin-resistant enterococcal infections: Epidemiology, clinical manifestations, and optimal management. Infect Drug Resist. 2015. 8: 217-30
17. Pintado V, Cabellos C, Moreno S, Meseguer MA, Ayats J, Viladrich PF. Enterococcal meningitis: A clinical study of 39 cases and review of the literature. Medicine (Baltimore). 2003. 82: 346-64
18. Shihadeh F, Reed V, Faderl S, Medeiros LJ, Mazloom A, Hadziahmetovic M. Cytogenetic profile of patients with acute myeloid leukemia and central nervous system disease. Cancer. 2012. 118: 112-7
19. Stewart DJ, Keating MJ, McCredie KB, Smith TL, Youness E, Murphy SG. Natural history of central nervous system acute leukemia in adults. Cancer. 1981. 47: 184-96