- Department of Neurosurgery, The Ohio State University Wexner Medical Center, Columbus, Ohio.
DOI:10.25259/SNI_528_2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Asad S. Akhter, Ahmed Mohyeldin, Andrew J. Grossbach. Acute presentation of spinal gouty arthritis: A case report and review of literature. 29-Nov-2019;10:232
How to cite this URL: Asad S. Akhter, Ahmed Mohyeldin, Andrew J. Grossbach. Acute presentation of spinal gouty arthritis: A case report and review of literature. 29-Nov-2019;10:232. Available from: http://surgicalneurologyint.com/surgicalint-articles/9776/
Abstract
Background: Gout is an inflammatory arthritis that results from faulty purine metabolism, affecting approximately 4% of adults in the US, and predominately affects people in the fourth decade of life. Further, spinal gout is rarely the first presentation of gout, especially in younger individuals.
Case Description: A 26-year-old male came to the emergency room with acute lower extremity numbness and weakness. The MR demonstrated an enhancing epidural lesion at T6–T8 in the mid-thoracic spine. He subsequently underwent a decompressive laminectomy and fusion at levels T6–T9, resulting in full recovery 1 year later. The pathology demonstrated needle-like monosodium urate crystals consistent with the diagnosis of spinal gouty arthritis.
Conclusion: Gout rarely initially presents in a young adult in the spine. Here, we reviewed the case of spinal gout in a 26-year-old male who successfully underwent spinal surgery.
Keywords: Gout, Paralysis, Spinal cord injury, Spine
INTRODUCTION
Gout, that afflicts approximately 4% of adults in the United States, is an inflammatory arthritis that results from faulty purine metabolism.[
CASE REPORT
Clinical findings
A 26-year-old male presented with 1–2 months of progressive right lower extremity weakness culminating in a 1-day history of the inability to ambulate. On examination, he exhibited 4-/5 hip flexion, knee extension, and knee flexion bilaterally in the lower extremities, 2/5 right-sided plantar flexor weakness, left lower extremity anteromedial hip numbness, and patellar hyperreflexia.
Radiographic presentation
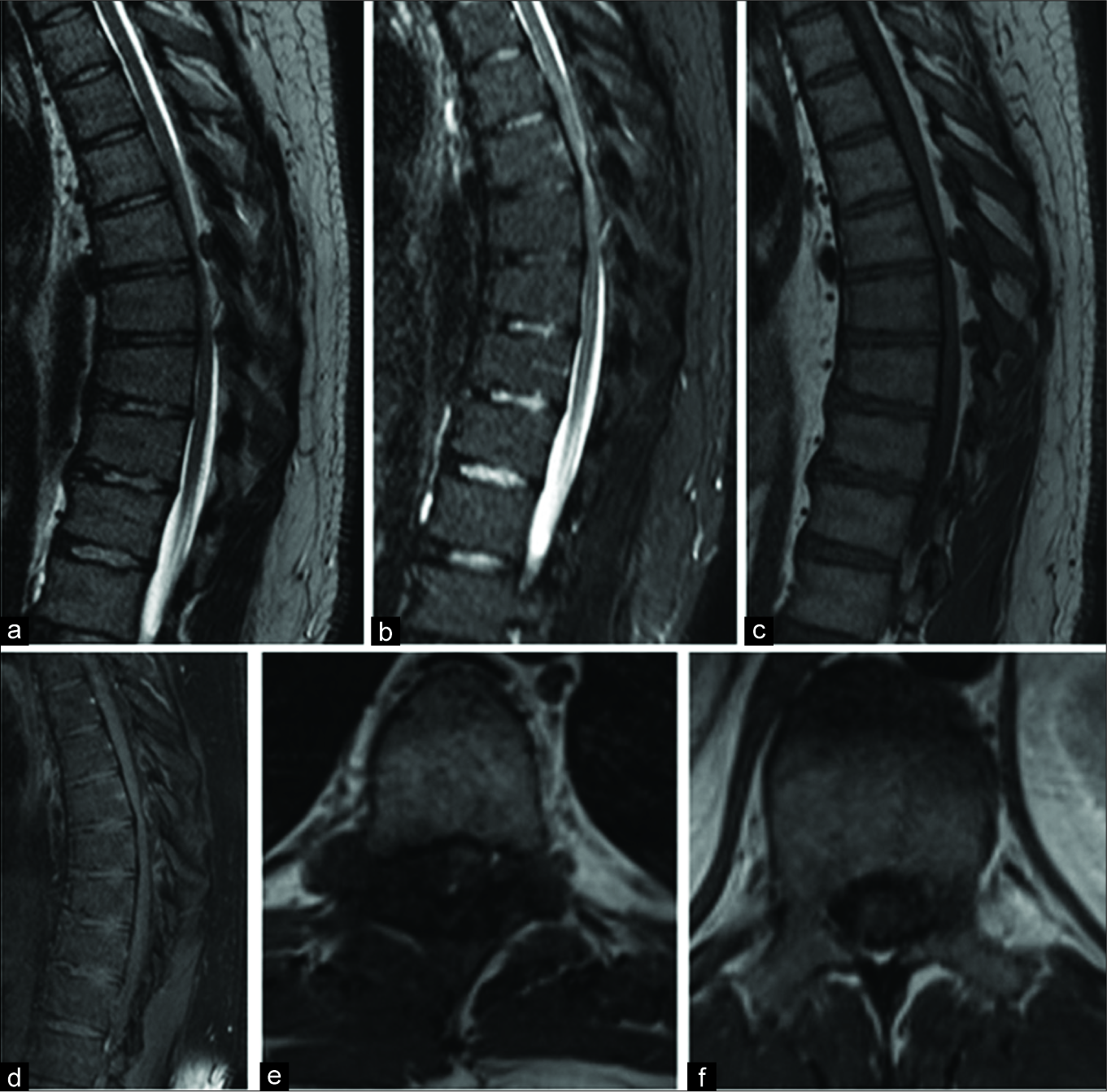
The thoracic MR with and without contrast demonstrated a right posterior-lateral T2 enhancing epidural lesion extending from T6 to T8 contributing to severe canal/cord stenosis and mild cord T6–T9 cord edema [
Figure 1:
Magnetic resonance imaging (MRI) images with the right lateral epidural abnormality in the mid-thoracic spine with spinal cord compression. (a) Sagittal T2 sequences, (b) sagittal short-tau inversion recovery sequences, (c) sagittal T1 sequences without contrast, (d) sagittal T1 sequences with contrast, (e) axial T1 MRI with contrast demonstrating extension of a tophus into the spinal canal and compressing the spinal cord to the left, (f) axial T1 magnetic resonance imaging with contrast at a level with no inflammatory or degenerative changes and no spinal cord compression.
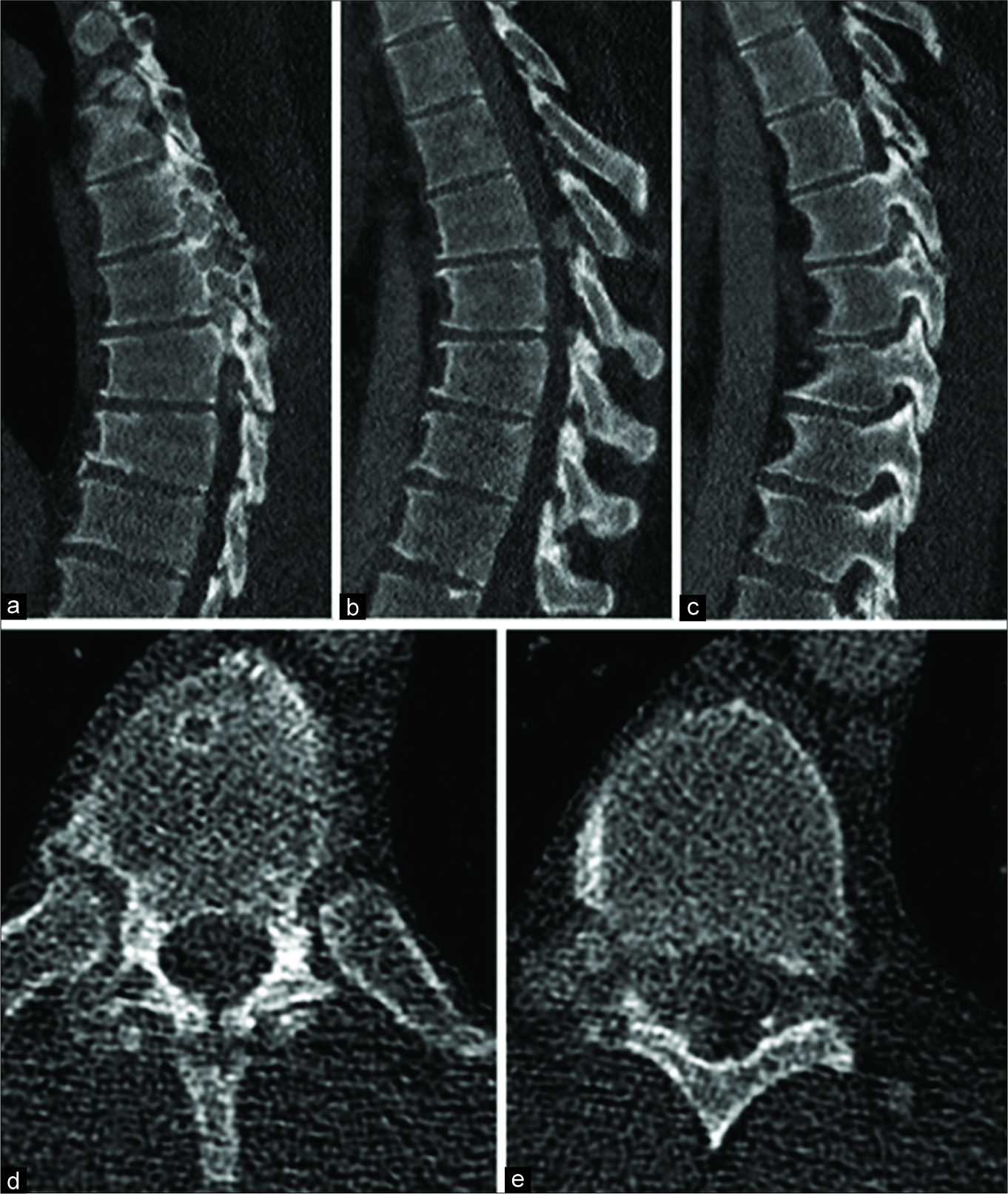
Figure 2:
Computed tomography (CT) images showing lytic process of bilateral thoracic facet joins with compression of the spinal cord. (a) Sagittal CT scans of the mid-thoracic spine demonstrating erosive changes of the right facet joints, (b) sagittal STIR sequences, (c) sagittal T1 sequences without contrast, (d) sagittal T1 sequences with contrast, (e) axial T1 magnetic resonance imaging with contrast demonstrating extension of a tophus into the spinal canal and compressing the spinal cord to the left.
Surgery
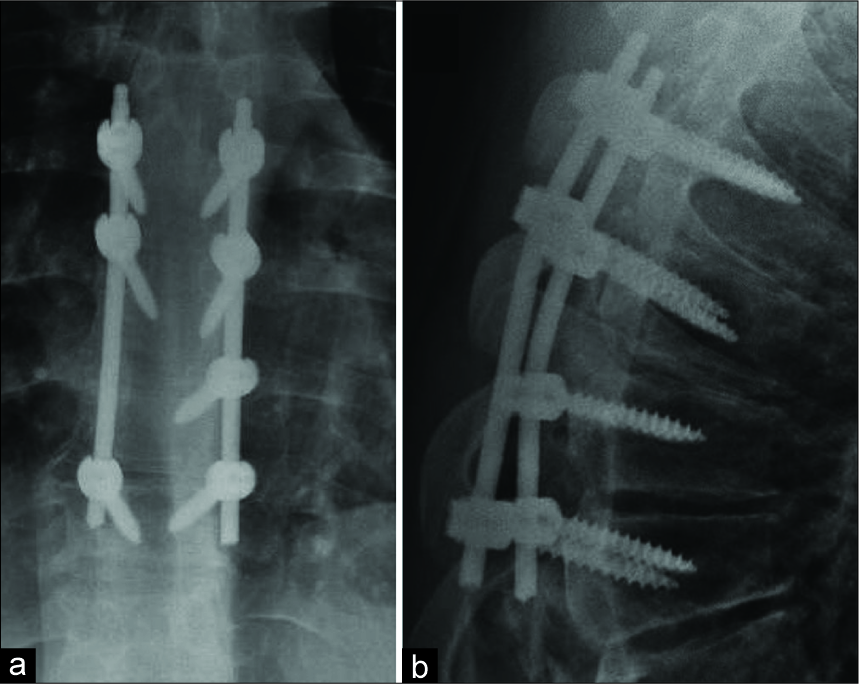
The next day, he underwent a T6–T9 decompressive laminectomy for the resection of the epidural mass accompanied by pedicle screw instrumentation [
Postoperative course
On postoperative day 1, the patient was 4+/5 in the lower extremities bilaterally, and the numbness of the anteromedial segment of his left lower extremity was markedly improved. He was discharged 6 days later, and at 1 postoperative year, his neurological examination was normal.
Pathology
The final pathology demonstrated whitish aggregates of strand-like material surrounded by inflammatory cells, including many foreign body giant cells. Under polarized light, these aggregates revealed strongly negative birefringent needle-shaped crystals consistent with gout.
Further, the postoperative rheumatologic workup documented elevated serum and urine uric acid levels. In addition, an X-ray of the right hand showed a synovial inflammatory process involving the ring finger. The patient was started on colchicine 0.6 mg b.i.d. and allopurinol 200 mg q.d.
DISCUSSION
Here, we described a rare case of spinal tophaceous gout in a 26-year-old male who newly presented with an acute paraparesis. The diagnosis of gout was not originally entertained, given the patient’s negative history and young age.
In a review of 131 cases, Toprover et al. found that gout can affect the lumbar (38%), cervical (24.8%), and thoracic spine (17.8%).[
The current epidemiologic studies show that the prevalence of gout in patients between the ages of 18 and 44 is <0.3%.[
CONCLUSION
Here, we presented the rare scenario of a 26-year-old male acutely developing paraparesis secondary to T6–T9 tophaceous gout.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Draganescu M, Leventhal LJ. Spinal gout: Case report and review of the literature. J Clin Rheumatol. 2004. 10: 74-9
2. Dwarki K, Dothard A, Abadie B, Miles MC. Rogue one: A story of tophaceous gout in the spine. BMJ Case Rep. 2018. 2018: bcr-2017-221163
3. Toprover M, Krasnokutsky S, Pillinger MH. Gout in the spine: Imaging, diagnosis, and outcomes. Curr Rheumatol Rep. 2015. 17: 70-
4. Wallace KL, Riedel AA, Joseph-Ridge N, Wortmann R. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol. 2004. 31: 1582-7
5. Wang W, Li Q, Cai L, Liu W. Lumbar spinal stenosis attributable to tophaceous gout: Case report and review of the literature. Ther Clin Risk Manag. 2017. 13: 1287-93
6. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: The national health and nutrition examination survey 2007-2008. Arthritis Rheum. 2011. 63: 3136-41