- Department of Neurosurgery, Tokyo Women’s Medical University Adachi Medical Center, Tokyo, Japan.
Correspondence Address:
Hidenori Ohbuchi, Department of Neurosurgery, Tokyo Women’s Medical University Adachi Medical Center, Tokyo, Japan.
DOI:10.25259/SNI_553_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kohei Oyamada, Hidenori Ohbuchi, Kae Nishiyama, Daisuke Imazato, Mayuko Inazuka, Shinji Hagiwara, Yuichi Kubota. Acute subdural hematoma associated with disruption of tumor vessels due to rapid growth of falx meningioma: A case report and literature review. 28-Oct-2022;13:495
How to cite this URL: Kohei Oyamada, Hidenori Ohbuchi, Kae Nishiyama, Daisuke Imazato, Mayuko Inazuka, Shinji Hagiwara, Yuichi Kubota. Acute subdural hematoma associated with disruption of tumor vessels due to rapid growth of falx meningioma: A case report and literature review. 28-Oct-2022;13:495. Available from: https://surgicalneurologyint.com/surgicalint-articles/11959/
Abstract
Background: Meningiomas associated with acute subdural hematoma (ASDH) are rare. The rapid growth of meningiomas has been shown to be one of the mechanisms underlying bleeding. We report the first case of ASDH during an imaging follow-up for the rapid growth of a falx meningioma.
Case Description: A 77-year-old woman was diagnosed with an incidental tumor along the right falx cerebri 3 years before bleeding. The follow-up magnetic resonance imaging (MRI) after 3 years showed that the tumor volume had rapidly increased from 4.31 cm3 to 22.27 cm3. The blood vessels around the tumor were stretched. The patient was scheduled to undergo tumor removal surgery. However, the patient experienced a sudden onset of disturbance of consciousness and was transferred to our hospital. On arrival, her Glasgow Coma Scale (GCS) score was 6 (E1V1M4) and right hemiplegia was observed. The patient had no history of traumatic events. Computed tomography (CT) showed left hemispheric and interhemispheric ASDH. Digital subtraction angiography revealed neither tumor staining nor abnormal vessels. Gross total tumor removal and hematoma evacuation were performed. There were no obvious active intraoperative bleeding points. The pathologic diagnosis was meningioma, the World Health Organization Grade I. Postoperative course revealed a GCS score of 10 (E4V1M5) and she was transferred to a rehabilitation hospital.
Conclusion: The disruption of tumor vessels due to the rapid growth of meningiomas may be a cause of bleeding. Incidental falx meningiomas with stretched tumor vessels due to rapid growth could indicate the need for early surgery.
Keywords: Acute subdural hematoma, Falx meningioma, Hemorrhage, Meningioma, Rapid growth
INTRODUCTION
Meningiomas are benign tumors and the most common central nervous system tumors in adults.[
CASE DESCRIPTION
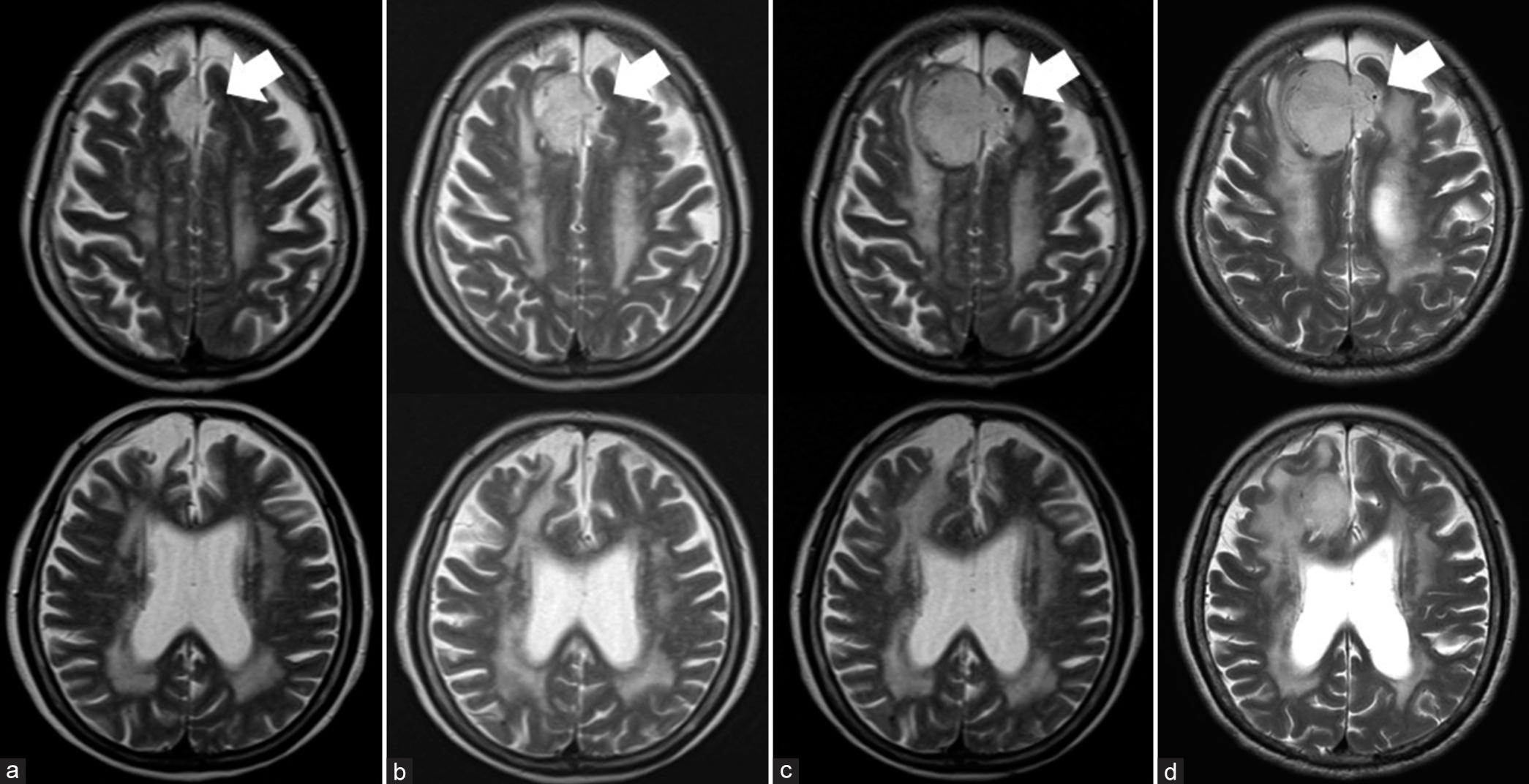
A 77-year-old woman with a history of cerebral infarction and chronic low back pain was taking antiplatelet drugs and selective serotonin reuptake inhibitors (SSRI). She had undergone magnetic resonance imaging (MRI) at another hospital 3 years before bleeding because of a headache, and an incidental tumor (volume 4.31 cm3) along the right falx cerebri had been identified. MRI showed the tumor as a well-circumscribed, slightly high-intensity area on T2-weighted images and a dural tail sign. The tumor was suspected to be a falx meningioma. The follow-up MRI 1 year before bleeding showed that the tumor had rapidly grown to 12.76 cm3. The follow-up MRI 2 weeks before bleeding showed 22.27 cm3 of volume and grown not only to the right but also to the left falx cerebri. Furthermore, MRI revealed a left flow void showing blood vessels that had moved from the median side to the lateral side due to tumor growth [
Figure 1:
Follow-up MRI scans; white arrows indicate flow voids on the left side of the tumor corresponding to adjacent vessels: (a) initial MRI images obtained 3 years before bleeding. Tumor volume: 4.31 cm3, (b) second follow-up MRI images obtained 1 year before bleeding. Tumor volume: 12.76 cm3, (c) third follow-up MRI images obtained 2 months before bleeding. Adjacent blood vessels were stretched and moved to the left due to rapid tumor growth. Tumor volume: 21.67 cm3, and (d) fourth follow-up MRI images obtained 2 weeks before bleeding. Tumor volume: 22.27 cm3.
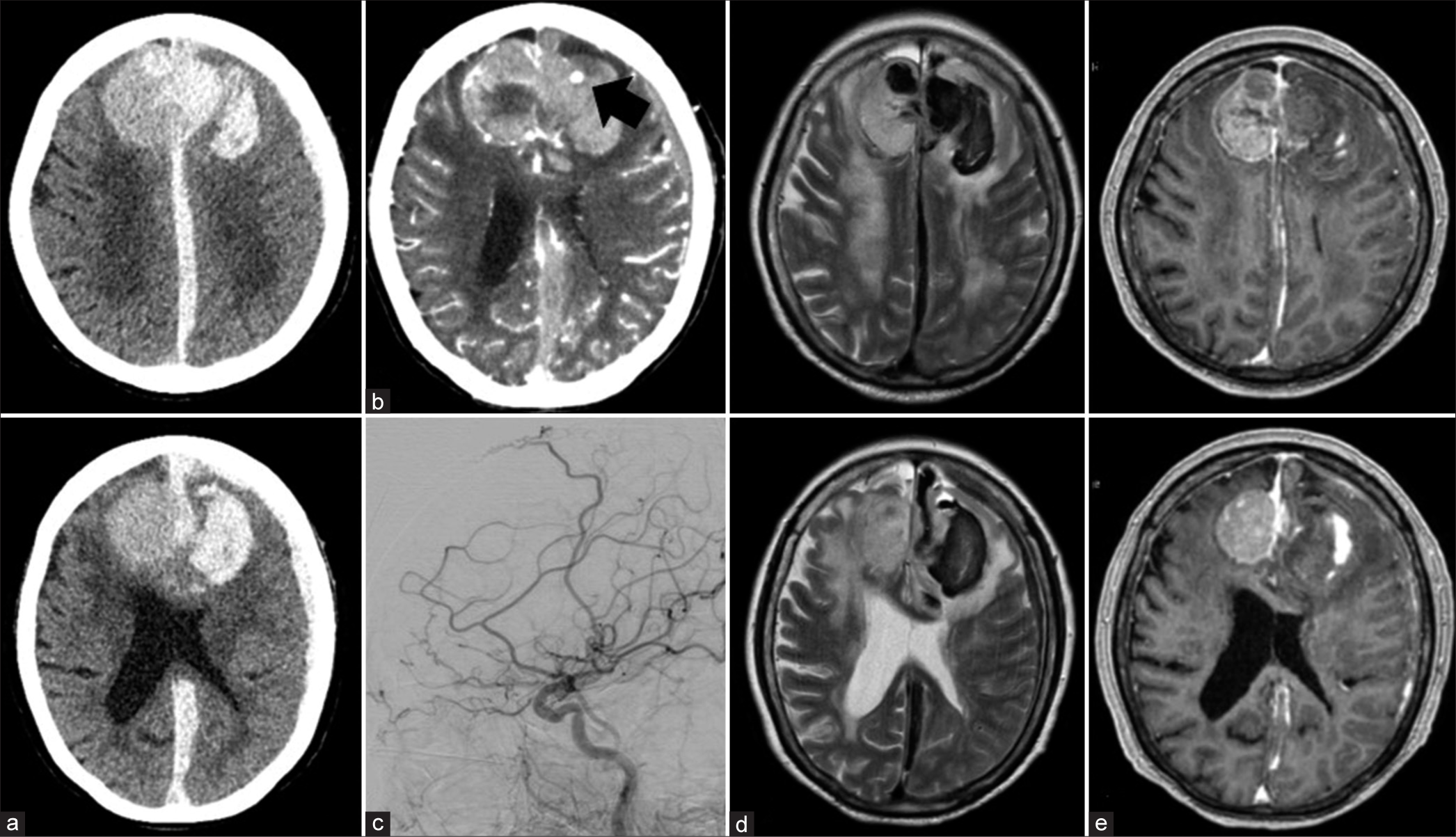
Figure 2:
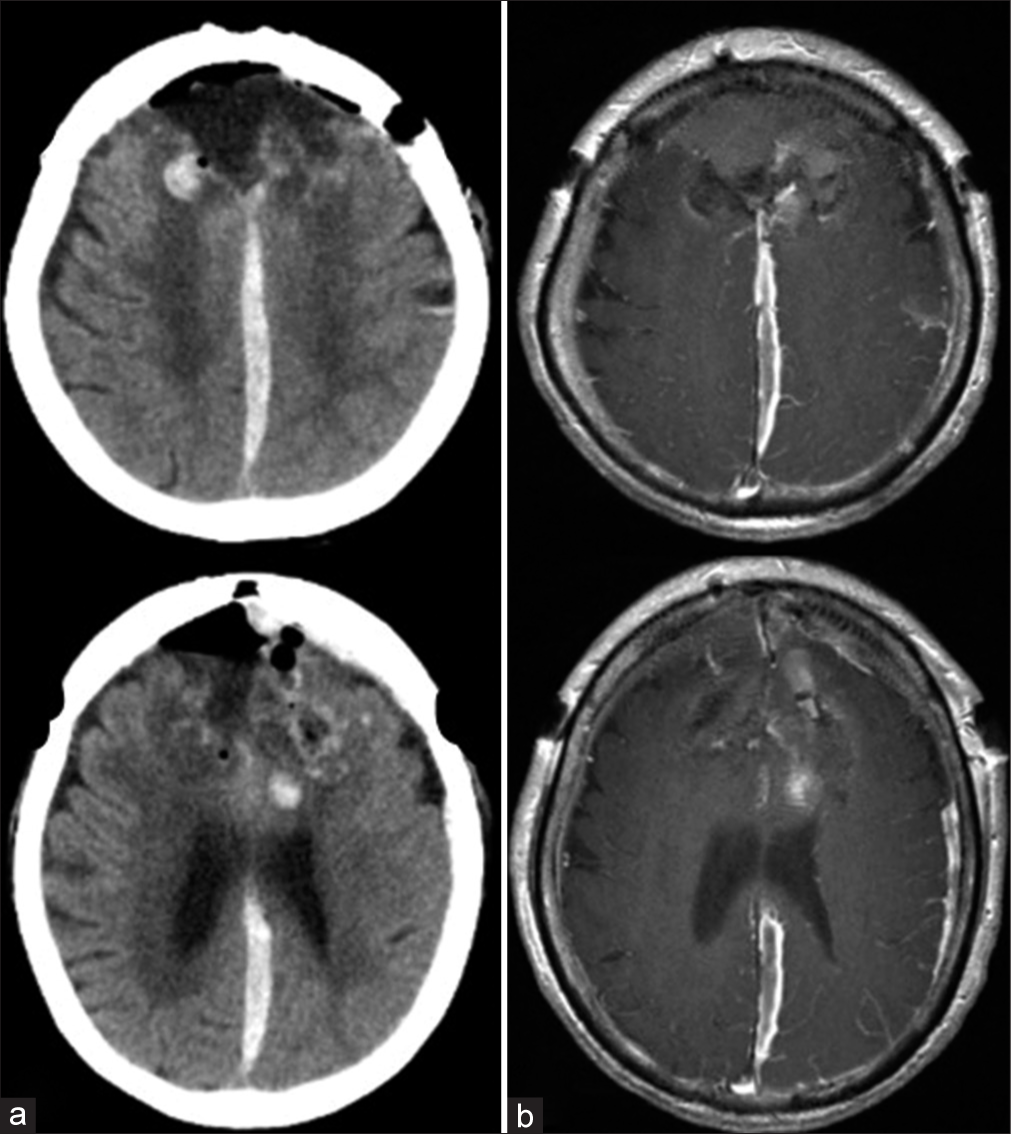
Imaging at onset of bleeding: (a) the head CT images at onset of bleeding showed left acute subdural hematoma (ASDH), interhemispheric ASDH, and intracerebral hematoma extending from the left intra-/extra-tumoral lesion. (b) Enhanced CT showing extravasation (black arrow) in the hematoma of the left frontal lobe. (c) Left lateral DSA of the left CCA revealed neither tumor staining nor abnormal vessels. (d and e) The mass lesion on the MRI image shows a mixed signal intensity area on T2-weighted images.
Tumor removal and hematoma evacuation were performed 3 days after admission. First, we removed the left frontal hematoma due to an increase in dural pressure and subsequently confirmed a decrease in dural pressure. Second, we performed internal decompression of the tumor and incised the SSS and falx to block feeding arteries. Third, we dissected the tumor from the surrounding normal brain parenchyma, removed the gross total of the tumor together with the falx of the attachment (Simpson Grade I), and drained the hematoma. No clear active bleeding points or intratumoral subacute clot was found intraoperatively. Fragile and irregular vessels were attached to the tumor wall.
The pathological diagnosis was confirmed as meningothelial meningioma (the World Health Organization [WHO] Grade I according to the 2016 Classification of Tumors of the Central Nervous System[
DISCUSSION
Meningiomas accompanied by subdural hematoma are uncommon.[
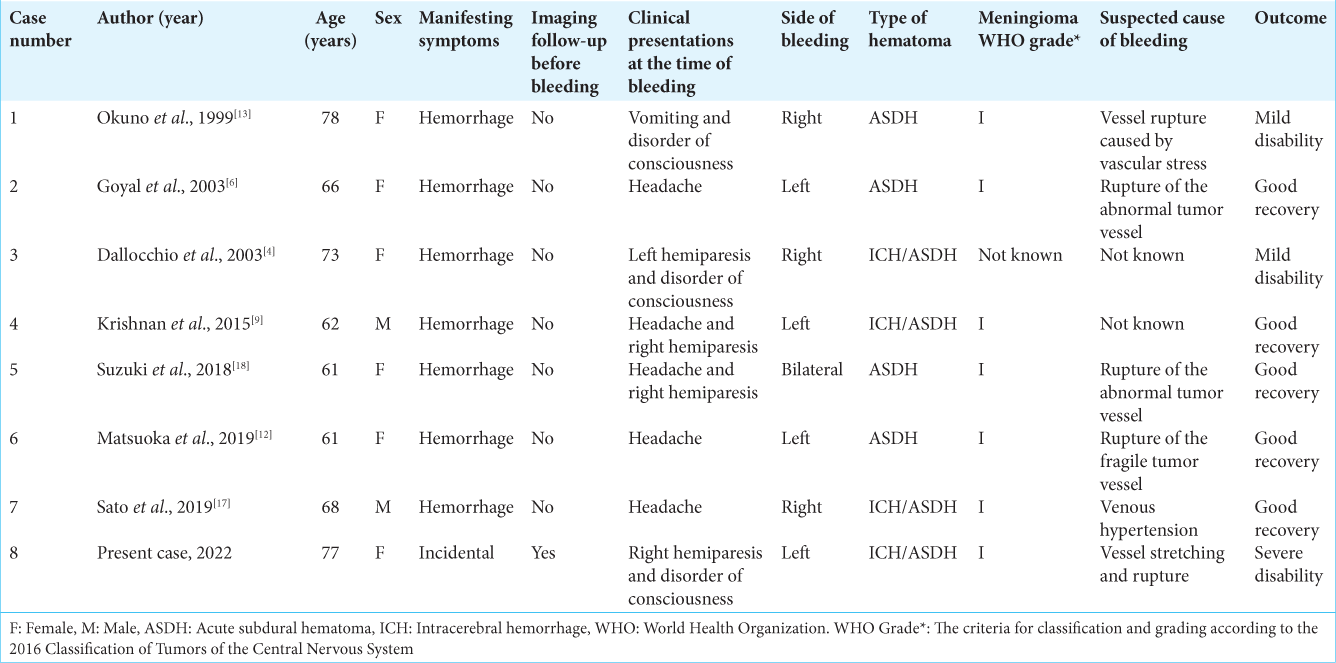
In five of the eight cases, the cause was bleeding due to suspected disruption of the tumor vessels, but this case was the only one showing rapid growth in imaging follow-up. The patient’s prognosis was generally good. However, when massive bleeding was observed at the time of admission, as in this case, the prognosis could be poor.
Several hypotheses have been proposed as the mechanism for the rapid growth of meningiomas accompanied by massive ASDH: (1) disruption of fragile tumor vessels by rapid growth of tumors; this mechanism was most commonly reported,[
Previously, rapid growth was reported to occur in 16–25% of meningiomas.[
In this case, the tumor was not a pathologically malignant meningioma and had no apparent traumatic mechanism. Intraoperative findings revealed stretched fragility and irregularity of the vessels in the tumor wall. Moreover, from a retrospective point of view, the follow-up image before bleeding confirmed that the blood vessels around the tumor had stretched due to the rapid growth of the tumor. On the contralateral bleeding side, the tumor rapidly grew and was large but not bleeding. It was considered that there were no stretched fragile vessels around the tumor. Contrast CT immediately after bleeding showed extravasation at the same site as the stretching blood vessels. No obvious vascular abnormalities were observed on angiography. In this case, during the rapid growth of the tumor confirmed by imaging follow-up, disruption of the stretched blood vessels led to bleeding, which is consistent with previous assumptions.
Recent studies showed that the risk factors for hemorrhage from meningioma were age, location, malignant histopathology, presence of hypertension, anticoagulant or antiplatelet use, and Serotonin Noradrenaline Reuptake Inhibitor (SNRI) use.[
CONCLUSION
We report the first case of ASDH due a falx meningioma that showed rapid growth during imaging follow-up. This was speculated to be caused by the disruption of stretched tumor vessel due to the rapid growth of the falx meningioma. Patients at high risk of bleeding are prone to massive hematomas and might have a poor prognosis. Incidental falx meningiomas with stretched tumor vessels due to rapid growth suggest that early surgery may be beneficial.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Grant support
None. The authors have no personal or institutional financial interests in drugs, materials, or devices described in this paper.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Abi Jaoude S, Peyre M, Degos V, Goutagny S, Parfait B, Kalamarides M. Validation of a scoring system to evaluate the risk of rapid growth of intracranial meningiomas in neurofibromatosis Type 2 patients. J Neurosurg. 2021. 134: 1377-85
2. Arbelaez A, Castillo M, Armao D. Meningioma presenting as an acute subdural hematoma. Emerg Radiol. 1999. 6: 149-52
3. Bosnjak R, Derham C, Popović M, Ravnik J. Spontaneous intracranial meningioma bleeding: Clinicopathological features and outcome. J Neurosurg. 2005. 103: 473-84
4. Dallocchio C, Borutti G, Frascaroli G, Camana C, Magrotti E. A sudden bleeding meningioma. Neurol Sci. 2003. 23: 327-28
5. Fathi AR, Roelcke U. Meningioma. Curr Neurol Neurosci Rep. 2013. 13: 337
6. Goyal A, Singh AK, Kumar S, Gupta V, Singh D. Subdural hemorrhage associated with falcine meningioma. Neurol India. 2003. 51: 419-21
7. Hashiba T, Hashimoto N, Izumoto S, Suzuki T, Kagawa N, Maruno M. Serial volumetric assessment of the natural history and growth pattern of incidentally discovered meningiomas. J Neurosurg. 2009. 110: 675-84
8. Huang R, Su S, Yang Z, Wang H, Hong L, Chen L. Neuroradiologic findings and clinical features of meningiomas with spontaneous hemorrhage onset: A single center 10-year experience. World Neurosurg. 2022. 162: e605-15
9. Krishnan P, Jena M, Kartikueyan R. Occult falcine meningioma unmasked following nearly complete hemorrhagic transformation with resultant spontaneous acute interhemisphelic subdural hematoma. J Neurosci Rural Pract. 2015. 6: 91-3
10. Lee EJ, Kim JH, Park ES, Kim YH, Lee JK, Hong SH. A novel weighted scoring system for estimating the risk of rapid growth in untreated intracranial meningiomas. J Neurosurg. 2017. 127: 971-80
11. Louis DN, Perry A, Reifenberger G, von Deimling A, FigarellaBranger D, Cavenee WK. The 2016 world health organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016. 131: 803-20
12. Matsuoka G, Eguchi S, Ryu B, Tominaga T, Ishikawa T, Yamaguchi K. Treatment strategy for recurrent hemorrhage from meningioma: Case report and literature review. World Neurosurg. 2019. 124: 75-80
13. Okuno S, Touho H, Ohnishi H, Karasawa J. Falx meningioma presenting as acute subdural hematoma: Case report. Surg Neurol. 1999. 52: 180-4
14. Omar AT, Starreveld YP. Meningioma associated with subdural hematoma: A systematic review of clinical features and outcomes. World Neurosurg. 2022. 158: e465-75
15. Pressman E, Penn D, Patel NJ. Intracranial hemorrhage from meningioma: 2 novel risk factors. World Neurosurg. 2020. 135: 217-21
16. Russell NA, Al-Fayez N, Joaquin AJ. Acute subdural hematoma with intracranial meningioma. Ann Saudi Med. 1993. 13: 369-71
17. Sato K, Matsuo A, Hiu T, Kurohama H, Miura S, Ito K. Falx meningioma presenting with intratumoral hemorrhage and interhemisphelic acute subdural hematoma: A case of report. No Shinkei Geka. 2020. 48: 413-22
18. Suzuki Y, Fujimoto M, Kawakita F, Asakura F, Murata H, Morooka Y. Tiny falx meningioma causing massive interhemishelic subdural hematoma: A case report. NMC Case Rep J. 2018. 5: 51-5