- Department of Pediatric Neurosurgery, Dana Children's Hospital, Tel-Aviv Medical Center, Tel-Aviv, Israel
- Department of Neurosurgery, Tel-Aviv Medical Center, Tel-Aviv, Israel
- Tel-Aviv University, Tel-Aviv, Israel
Correspondence Address:
Jonathan Roth
Department of Pediatric Neurosurgery, Dana Children's Hospital, Tel-Aviv Medical Center, Tel-Aviv, Israel
Department of Neurosurgery, Tel-Aviv Medical Center, Tel-Aviv, Israel
Tel-Aviv University, Tel-Aviv, Israel
DOI:10.4103/sni.sni_338_18
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Assaf Berger, Shlomi Constantini, Zvi Ram, Jonathan Roth. Acute subdural hematomas in shunted normal-pressure hydrocephalus patients – Management options and literature review: A case-based series. 28-Nov-2018;9:238
How to cite this URL: Assaf Berger, Shlomi Constantini, Zvi Ram, Jonathan Roth. Acute subdural hematomas in shunted normal-pressure hydrocephalus patients – Management options and literature review: A case-based series. 28-Nov-2018;9:238. Available from: http://surgicalneurologyint.com/surgicalint-articles/9096/
Abstract
Background:Ventriculoperitoneal shunting (VPS) is considered a risk factor for developing subdural hematomas (SDH). Treating cases of acute SDH (aSDH) in shunted normal-pressure hydrocephalus (NPH) patients can be challenging, and data in this field are scarce. We report our experience treating shunted NPH patients presenting with aSDH.
Methods:Eight patients, aged 73 ± 6 years, with a history of VPS for NPH, hospitalized because of aSDH were included in this study. We retrospectively analyzed data regarding patients’ clinical and radiological presentation, hospitalization course, the use of antithrombotics, and response to different treatment regimens.
Results:Four patients had pure aSDH, three had acute on chronic SDH, and one had subacute SDH. Patients presented with GCS 13–15 and various neurological signs, mainly confusion and unsteady gate. Two cases improved following resetting of their programmable shunt valve to its maximal pressure setting. Six cases improved after evacuation of the hematomas, five of them were operated a few days after initially resetting of the valve pressure. Three patients were discharged home, whereas five were referred to rehabilitation. Extended Glasgow Outcome Scale scores at discharge and during long-term follow-up were 5 and 7, respectively.
Conclusions:Treatment of patients with VPS for NPH, presenting with aSDH, may differ according to the neurological status, imaging, and clinical course. Treatment options include restricting shunt function, hematoma evacuation, or both.
Keywords: Acute subdural hematoma, hydrocephalus, shunt, treatment
INTRODUCTION
Normal-pressure hydrocephalus (NPH) is characterized by the Hakim–Adams triad, including gait disturbances, memory decline, and urinary incontinence. Ventriculoperitoneal shunts (VPS) are commonly used for treatment of NPH.[
Managing cases of acute SDH (aSDH) in shunted NPH patients can be challenging because of the need for simultaneously addressing not only the hematoma itself but also the draining function of the shunt. The literature regarding handling of aSDH in patients with shunts is scarce and mainly based on case reports or small series.[
As the data regarding this topic are limited, we chose to present our experience in treating NPH patients with VPS, who suffered from aSDH. An analysis and comparison of the different treatment options are discussed.
MATERIALS AND METHODS
We retrospectively reviewed the hospital records of adult (>18 years old) NPH patients with VPS, who suffered from aSDH, that were hospitalized in the Department of Neurosurgery at the Tel Aviv Medical Center between the years 2010 and 2016. We included only primary NPH patients. Patients with acute on chronic or subacute bleeds were included too.
We did not include cases who presented with purely chronic bleeds or hypodense subdural effusions. Patients with intraparenchymal hematomas were also not included.
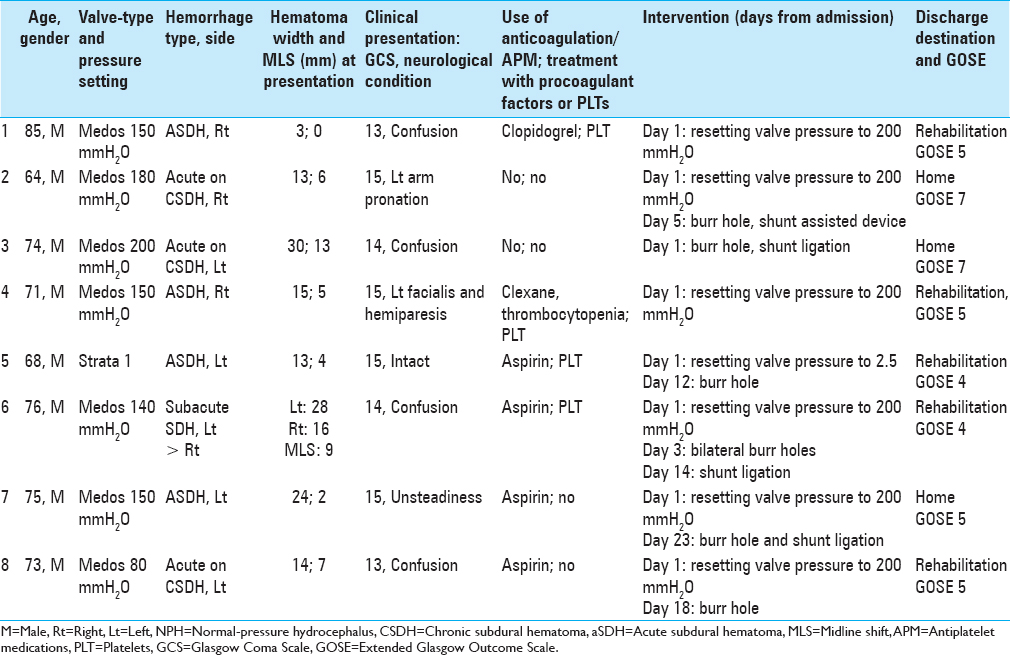
Eight patients with acute, acute on chronic, or subacute SDH were identified and analyzed for the following details: patients’ age, gender, cause of hydrocephalus, and use of APM or anticoagulant medications prior to injury Circumstances and timing of head injury, duration between symptom onset and admission, Glasgow Coma Scale (GCS), and neurological deficits on admission and during hospitalization were documented. The maximal width of the hematomas and midline shift (MLS) were measured on admission and during follow-up Treatments and their timing during the hospitalization were recorded, including changing of the shunt's valve pressure setting, shunt ligation or externalization, the addition of shunt assist devices, and surgical procedures for evacuation of the hematomas Outcome included discharge destination (home, rehabilitation, or nursing care) and Extended Glasgow Outcome Scale (GOSE) at discharge and follow-up.[
The study was performed following an Institutional Review Board approval (0705-16-TLV) and in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki. For this type of retrospective study, informed consent was waived. The authors have no potential conflicts of interest to disclose. This study did not receive any specific grants from funding agencies in the public, commercial, or not-for-public sectors.
Statistics
This is an observational study, focusing on a small patients group; thus, no statistical analyses were done. Data are presented in a descriptive manner, and numerical data are presented as mean ± standard deviation.
RESULTS
Patient background
Over a period of 7 years (2010–2016), 210 patients received a VPS for the diagnosis of NPH at our center. During the same period, eight of these patients were hospitalized with acute or subacute SDH. The average age at presentation was 73 ± 6 years, ranging from 64 to 85 years of age. All patients were male.
Five patients were pretreated with APM, including four with aspirin and one with clopidogrel. APM were discontinued before shunt placement for at least 10 days and resumed within 1 month after discharge. One additional patient who had a history of lymphoma and was treated with enoxaparin also suffered from thrombocytopenia during the traumatic event [
Patient history and baseline imaging
All patients had VPS inserted for 14 ± 15 months (range 3–48) prior to their current presentation. Two types of shunt valves were used: Medos programmable valves with siphonguard (Codman Inc.) programed to 80–200 mmH2O (seven cases) and Strata valve (Medtronic Inc.) programed at 1 (one case). All shunts were located in the right lateral ventricle and were placed via a parieto-occipital entry [
Baseline imaging, performed 5 months (range 2–11) prior to the current admission, showed no subdural bleeding. The indication for these CT scans was routine surveillance of shunted NPH patients to document radiological signs of overdrainage. Case 3 had bilateral subdural hygromas and had insertion of shunt-assisted device (SAD) because of overdrainage syndrome 2 months prior to developing acute on chronic SDH. This patient did not have a new CT scan following the addition of the SAD and prior to admission with the bleed. The other seven cases had no evidence for subdural collections prior to their current admission.
Presentation on admission
Four patients had an aSDH following a fall on the day of admission. Three patients had an acute on chronic SDH, and one had subacute SDH, most of which were associated with recurrent falls over a period of several weeks to months with an acute deterioration just before their admission. GCS on admission ranged from 13 to 15 and the examination was mainly significant for non-focal signs, such as confusion (4) and unsteady gait (1), but also hemiparesis (2) and facial palsy (1).
Hematomas were located on the left (contralateral to the shunt) in five patients (one of which had also a small right bleed). Maximal hematoma width and MLS were 30 and 13 mm, respectively, both of which occurred in an NPH patient with acute on chronic SDH. In cases of left-sided (contralateral) SDH, the size of hematoma and MLS on admission was 22 ± 8 and 7 ± 4 mm, respectively, as compared with 10 ± 6 and 4 ± 3 mm on the right ipsilateral side [
Management of clotting disturbances
Three of the five patients who were using APM, as well as one patient with lymphoma and thrombocytopenia that was also using enoxaparin, were given platelets on admission. In all cases, APM and anticoagulants were stopped on admission. Eventually, APM were discontinued permanently in two of the five previously treated patients, whereas it was recommenced in three of them. Enoxaparin treatment was recommenced before discharge for the prophylaxis of venous thrombosis in the case of lymphoma and thrombocytopenia [
Management
There were no urgent craniotomies for aSDH. Burr hole (BH) evacuation of SDH was performed in six cases on days 1–23 of admission. BH was performed in all five cases in which the hematoma was contralateral to the shunt, as compared with only one of three cases, in whom the hematoma was ipsilateral to the shunt.
Patients were treated along three different pathways: increasing shunt valve pressure settings without evacuation of the hematoma (two cases), early BH with concurrent shunt adjustments on day of hospitalization (one case), and shunt adjustments followed by late BH (five cases).
Increasing shunt valve pressure setting
Two cases (1 and 4) improved following resetting shunt valves to their maximal pressures. They later developed recurrent NPH symptoms and had their valve pressures decreased. Their MLS and hematoma thickness on admission were no more than 5 and 15 mm, respectively [
Early BH with concurrent shunt adjustments
One case (3) with acute on chronic SDH underwent a BH evacuation on the day of admission. This patient suffered from confusion and had a large hematoma of 30 mm and MLS of 13 mm. He showed substantial clinical improvement following BH and shunt ligation. One month later, he developed recurrent NPH-like symptoms necessitating reopening of the shunt system, with no need for any further interventions during a follow-up period of almost 2 years [
Shunt adjustments followed by late BH
Five cases (2, 5, 6, 7, 8) had their shunt valve pressures adjusted on admission but underwent BH between days 5 and 23 of admission. The initial size of the hematoma and MLS was relatively large, ranging 13–28 and 2–9 mm, respectively. Three patients had increase in either the hematoma size or MLS before the BH. Three patients had clinical deterioration, whereas two had no improvement under conservative treatment and thus had the hematoma drained. All patients improved upon discharge. Shunt valve pressure settings had to be later decreased in three cases because of recurrent NPH symptoms [
Outcome
Three patients were discharged home and five were referred to rehabilitation. No residual or recurrent hematoma was documented in any of the cases over a median of 10 months (range: 1 month–4 years). All patients showed improvement in their clinical outcome compared to that on admission. GOSE on discharge was 5 and improved to 7 after a few months of follow-up [
DISCUSSION
To our knowledge, this article is one of the first to describe management options in shunted NPH patients presenting with aSDH or subacuteSDH. In this article, we did not explore or analyze risk factors associated with bleed occurrence, but rather the actual treatment options. The typical patient would be an adult (usually an elderly), often taking anticoagulants or APM, presenting in the context of falls (with or without a clear head injury event), with a nonspecific neurological decline. Patients presenting with mass-effect-related symptoms – such as declined consciousness or focal neurological signs, necessitate an urgent hematoma evacuation with a craniotomy. However, smaller hematomas, or those with no mass effect-related symptoms, may be followed or treated with restriction of the shunt function. Some of these patients may eventually undergo hematoma evacuation at a later stage – possibly with a BH. Regardless to the treatment of the hematoma, restriction of the shunt's function was part of the treatment for all patients. Thus, we recommend restriction of the shunt function by increasing the shunt pressure settings. Whether to externalize the shunt and temporarily close it, ligate the shunt, or drain the hematoma, and the timing of such a procedure, should be based on clinical and radiological findings at presentation and during follow-up.
Reviewing the literature, there are limited data regarding the actual treatment of VPS patients presenting with aSDH, which is not associated with overdrainage. Yet, our decision-making for each individual case was based on previous reports and their relevance to the clinical scenarios.[
Interestingly, in our series, BH was more commonly done among patients whose hematomas were contralateral to the side of the shunt and were mostly located on the left hemisphere. Theoretically, patients with hematomas that are ipsilateral to the side of their shunts might benefit clinically from the shunt drainage that lowers the pressure on the side of the hematoma, thus decreasing its impact on brain parenchyma as well as MLS to the contralateral side, provoking less neurological changes. This theory may receive support by the fact that the sizes of SDH and MLS were bigger in the contralateral group, though not estimated statistically because of the small series size. However, it may of course result from the fact that left hemispheral bleeding causes more significant neurological changes by affecting critical functions on the dominant side, thus triggering more active interventions. Determination of the drainage side was not affected by reluctance to operate on the shunted side, but by clinical–radiological considerations.
The influence of long-term use of APM or anticoagulants on the risk of SDH among patients with VPS is a matter of debate. A recent report found a significantly increased rate of SDH among NPH patients who were using aspirin and underwent a VPS.[
As it is unclear whether APM actually increase the risk of intracranial hematoma expansion,[
Study limitations
The main limitations of our study are the small series size, as well as the retrospective nature of the study, which hamper the possibility of drawing any statistically based conclusions.
CONCLUSIONS
When treating aSDH in NPH-shunted patients, one should take into account the combined effects of both the hematoma mass effect and the CSF drainage by the shunt. Increasing the shunt valve pressure setting might be effective for some; however, these patients should be followed carefully as most will also require evacuation of their hematomas. Whether and when to evacuate the bleed are based on clinical and radiological findings and dynamics. Last, long-term follow-up is important, as some patients may need to readjust their shunt because of the recurrence of NPH-related symptoms.
Ethical approval
The study was performed following an Institutional Review Board (IRB) approval (0705-16-TLV) and in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Declaration of patient consent
For this type of retrospective study, informed consent was waived.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
None.
References
1. Baharoglu MI, Cordonnier C, Al-Shahi Salman R, de Gans K, Koopman MM, Brand A. Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): A randomised, open-label, phase 3 trial. Lancet (London, England). 2016. 387: 2605-13
2. Birkeland P, Lauritsen J, Poulsen FR. Aspirin is associated with an increased risk of subdural hematoma in normal-pressure hydrocephalus patients following shunt implantation. J Neurosurg. 2015. 123: 423-6
3. Birkeland P, Lauritsen J, Poulsen FR. Subdural haematoma complicating shunting for normal pressure hydrocephalus in the setting of concomitant antiplatelet medication-a report of 11 cases. Br J Neurosurg. 2016. 30: 567-70
4. Brean A, Eide PK. Prevalence of probable idiopathic normal pressure hydrocephalus in a Norwegian population. Acta Neurol Scand. 2008. 118: 48-53
5. Browd SR, Gottfried ON, Ragel BT, Kestle JR. Failure of cerebrospinal fluid shunts: Part II: Overdrainage, loculation, and abdominal complications. Pediatr Neurol. 2006. 34: 171-6
6. Frontera JA, Lewin JJ, Rabinstein AA, Aisiku IP, Alexandrov AW, Cook AM. Guideline for reversal of antithrombotics in intracranial hemorrhage: Executive summary. A statement for healthcare professionals from the Neurocritical Care Society and the Society of Critical Care Medicine. Crit Care Med. 2016. 44: 2251-7
7. Goodwin CR, Kharkar S, Wang P, Pujari S, Rigamonti D, Williams MA. Evaluation and treatment of patients with suspected normal pressure hydrocephalus on long-term warfarin anticoagulation therapy. Neurosurgery. 2007. 60: 497-501
8. Hayes J, Roguski M, Riesenburger RI. Rapid resolution of an acute subdural hematoma by increasing the shunt valve pressure in a 63-year-old man with normal-pressure hydrocephalus with a ventriculoperitoneal shunt: A case report and literature review. J Med Case Rep. 2012. 6: 393-
9. Hoh BL, Kleinhenz DT, Chi YY, Mocco J, Barker FG. Incidence of ventricular shunt placement for hydrocephalus with clipping versus coiling for ruptured and unruptured cerebral aneurysms in the Nationwide Inpatient Sample database: 2002 to 2007. World Neurosurg. 2011. 76: 548-54
10. Hoya K, Tanaka Y, Uchida T, Takano I, Nagaishi M, Kowata K. Treatment of traumatic acute subdural hematoma in adult hydrocephalus patients with cerebrospinal fluid shunt. Clin Neurol Neurosurg. 2012. 114: 211-6
11. Kamiryo T, Hamada J, Fuwa I, Ushio Y. Acute subdural hematoma after lumboperitoneal shunt placement in patients with normal pressure hydrocephalus. Neurol Med Chir (Tokyo). 2003. 43: 197-200
12. Khan QU, Wharen RE, Grewal SS, Thomas CS, Deen HG, Reimer R. Overdrainage shunt complications in idiopathic normal-pressure hydrocephalus and lumbar puncture opening pressure. J Neurosurg. 2013. 119: 1498-1502
13. Mahaney KB, Chalouhi N, Viljoen S, Smietana J, Kung DK, Jabbour P. Risk of hemorrhagic complication associated with ventriculoperitoneal shunt placement in aneurysmal subarachnoid hemorrhage patients on dual antiplatelet therapy. J Neurosurg. 2013. 119: 937-42
14. O’Kelly CJ, Kulkarni AV, Austin PC, Urbach D, Wallace MC. Shunt-dependent hydrocephalus after aneurysmal subarachnoid hemorrhage: Incidence, predictors, and revision rates. J Neurosurg. 2009. 111: 1029-35
15. Sundstrom N, Lagebrant M, Eklund A, Koskinen LD, Malm J. Subdural hematomas in 1846 patients with shunted idiopathic normal pressure hydrocephalus: Treatment and long-term survival. J Neurosurg. 2018. 129: 797-804
16. Weir J, Steyerberg EW, Butcher I, Lu J, Lingsma HF, McHugh GS. Does the extended Glasgow outcome scale add value to the conventional Glasgow outcome scale?. J Neurotrauma. 2012. 29: 53-8
17. Yamada SM, Tomia Y, Murakami H, Nakane M. Management for traumatic chronic subdural hematoma patients with well-controlled shunt system for hydrocephalus. Clin Case Rep. 2015. 3: 548-50