- Department of Neurosurgery, NTT Medical Center Tokyo, Shinagawa-ku, Tokyo, Japan.
DOI:10.25259/SNI_500_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sho Tsunoda, Tomohiro Inoue, Akihiro Shimoi, Atsuya Akabane. Acute surgery for a case of superior vermian arteriovenous malformation producing raised venous pressure coexisting with basilar-superior cerebellar artery aneurysm presenting subarachnoid hemorrhage; Case report. 20-Jan-2021;12:23
How to cite this URL: Sho Tsunoda, Tomohiro Inoue, Akihiro Shimoi, Atsuya Akabane. Acute surgery for a case of superior vermian arteriovenous malformation producing raised venous pressure coexisting with basilar-superior cerebellar artery aneurysm presenting subarachnoid hemorrhage; Case report. 20-Jan-2021;12:23. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10539
Abstract
Background: Superior vermian subtype of arteriovenous malformation (AVM) coexisting with proximal feeder aneurysm on basilar-superior cerebellar artery (BA-SCA) junction is an extremely rare situation. We experienced a case of this rare entity presenting with subarachnoid hemorrhage (SAH), and herein, introduce the outline and clinical features of this experience together with the actual surgical video.
Case Description: A 54-year-old man SAH patient with severe headache, disturbance of consciousness, and left oculomotor palsy was urgently admitted to our hospital. Imaging examination demonstrated superior vermian AVM with BA-SCA aneurysm, and both lesions were treated through two different approaches (left pterional craniotomy in conjunction with zygomectomy, and left posterior interhemispheric occipital transtentorial approach) in acute phase of SAH. Both lesions were completely disappeared postoperatively and the patient’s postoperative course was favorable, without symptomatic cerebral vasospasm. Although slight oculomotor palsy remained, the patient recovered well and was transferred to a rehabilitation hospital for further improvement.
Conclusion: In the cases of AVM coexisting with proximal feeder aneurysm, presenting with SAH, disorders of intracranial venous return associated with an AVM can be a vital hindrance to managing cerebral vasospasm; therefore, treating both lesions in the acute phase may lead to good outcomes.
Keywords: Arteriovenous malformation, Basilar artery aneurysm, Clipping, Occipital transtentorial approach, Subarachnoid hemorrhage
INTRODUCTION
Cerebellar arteriovenous malformation (AVM) has a relatively higher rupture rate than supratentorial AVM, and cerebellar AVM is difficult to treat because of its location surrounded by vital anatomical structures.[
In this study, we experienced an excellent outcome in a case of superior vermian AVM coexisting with a basilar-superior cerebellar aneurysm (BA-SCA) presenting with subarachnoid hemorrhage (SAH). In this case, we successfully performed radical two-stage operation for both lesions in the acute phase. In this report, we introduce the outline and clinical features of this experience, and present our methodology for completing safe surgery together with the actual surgical video.
CLINICAL PRESENTATION
A 54-year-old man presenting with abrupt severe headache, disturbance of consciousness with Glasgow coma scale (GCS) of 12 (E3V4M5), and left oculomotor palsy (ptosis of 3 mm, moderate exotropia, and anisocoria of 2 mm) was urgently transported and admitted to our hospital.
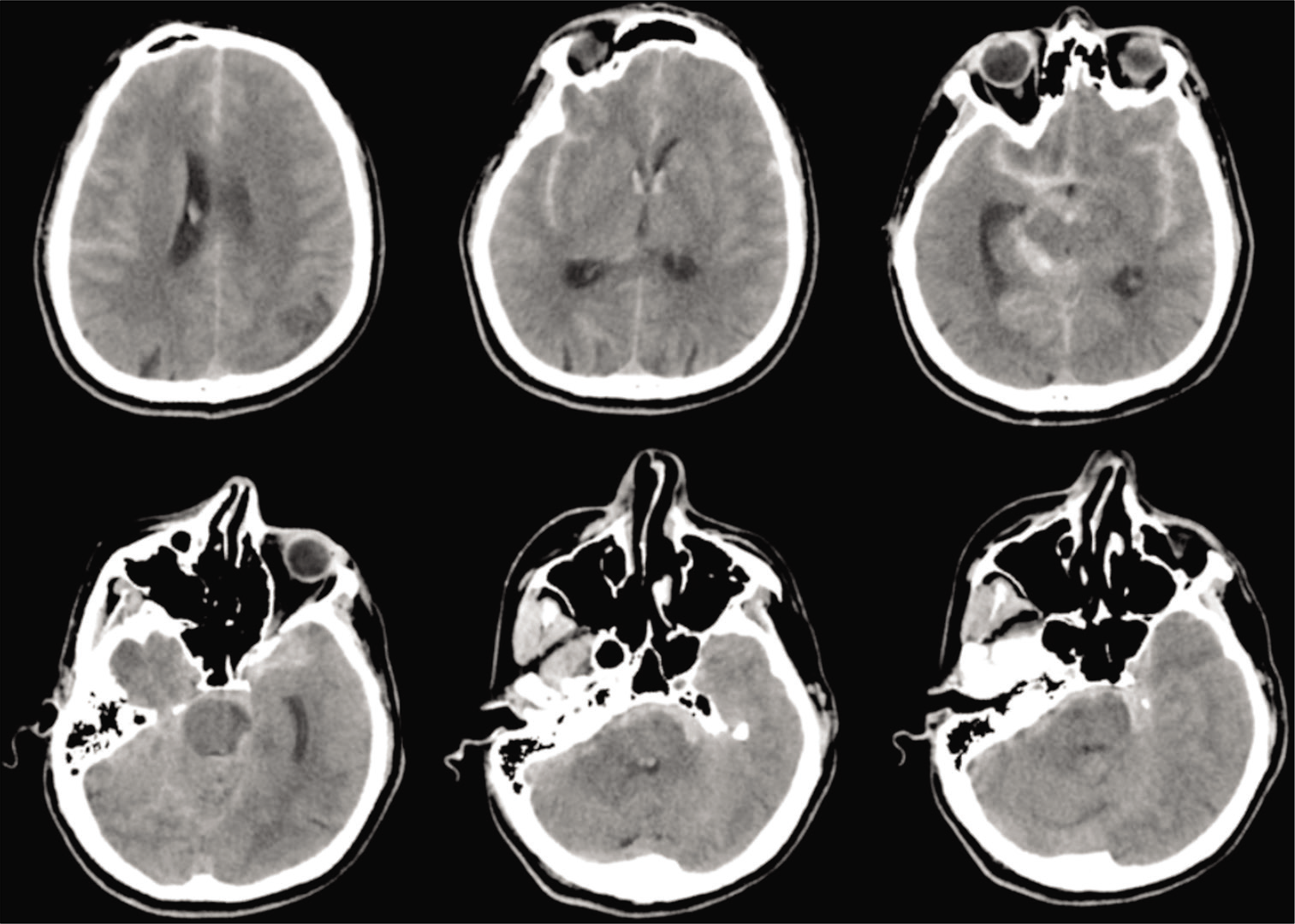
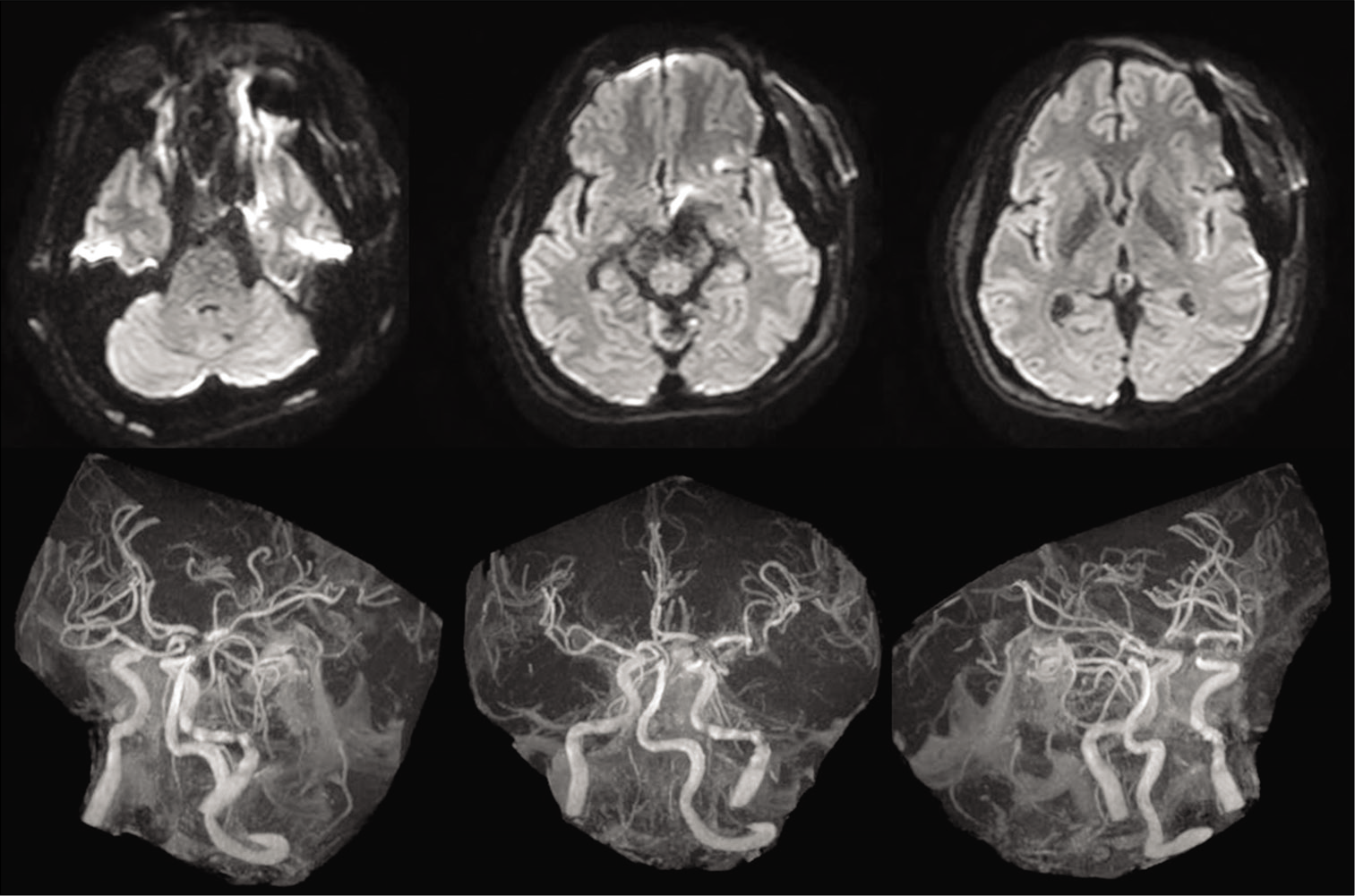
Computed tomography (CT) revealed diffuse SAH, particularly thickened at the right ambient to quadrigeminal cisterns [
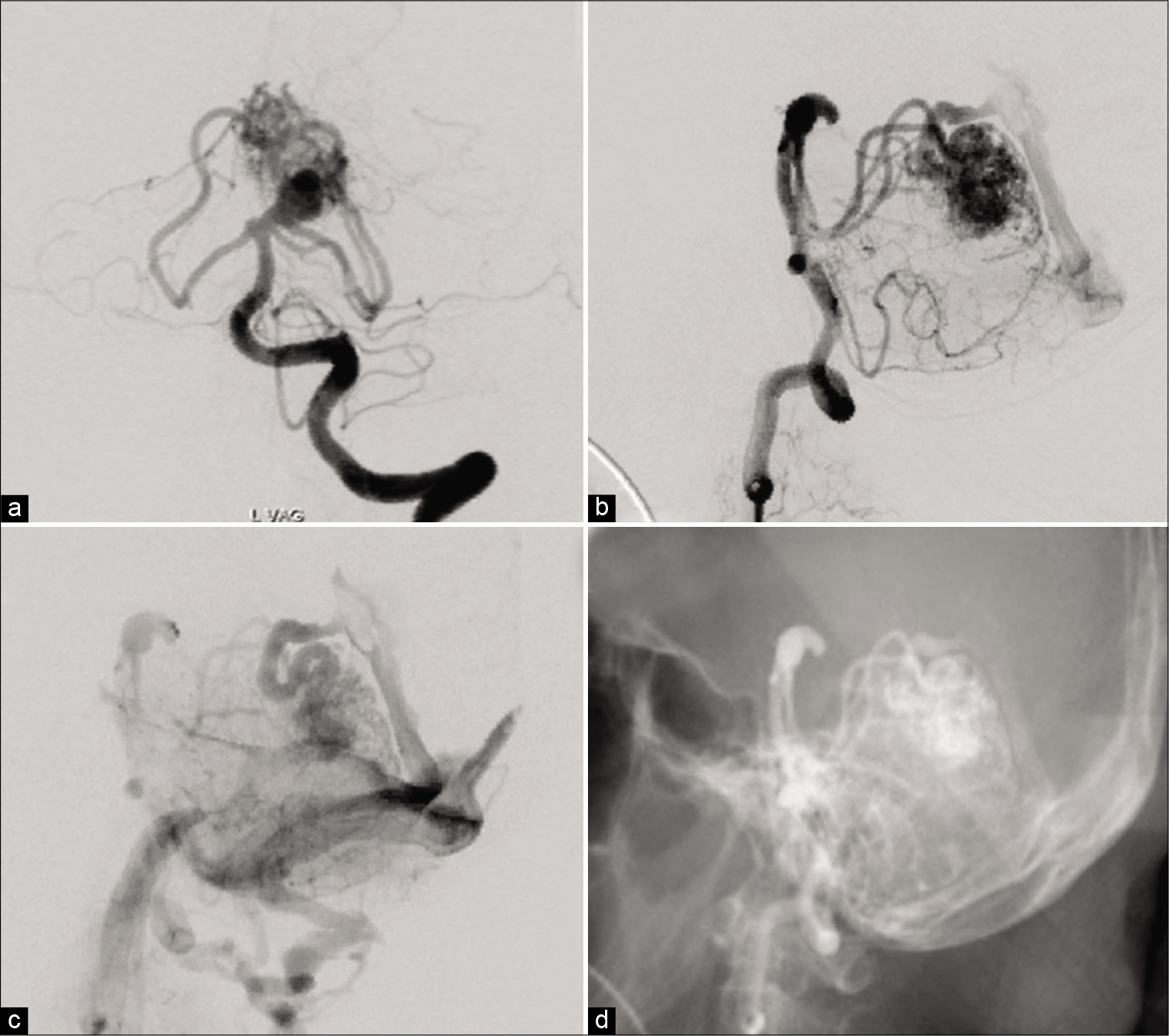
The AVM was mainly fed by two branches from the ipsilateral SCA in addition to the contralateral SCA and ipsilateral posterior inferior cerebellar artery (PICA) [
The aneurysm was located at the origin of the SCA, the main feeder of the AVM, and the dome and neck width were 10.5 mm and 4.1 mm, respectively. The aneurysmal neck and apex of the dome were located 5.0 mm and 12.1 mm above the clinoid line (connecting the anterior and posterior clinoid process), respectively [
We diagnosed SAH, World Federation of Neurological Surgeons Grading System for Subarachnoid Hemorrhage (WFNS) Grade 3. Imaging findings made it difficult to determine whether the aneurysm or AVM was the rupture site; however, the absence of intracranial hemorrhage (ICH) or intraventricular hemorrhage (IVH) and the presence of left oculomotor palsy suggested the possibility of aneurysmal rupture more than AVM rupture, and we prioritized treatment using aneurysmal clipping.
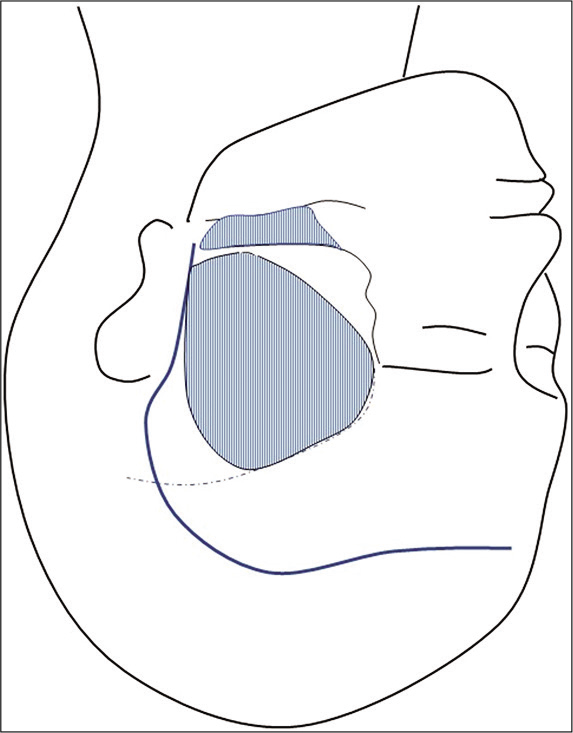
Considering the position of the neck and apex of the dome, we added zygomectomy in addition to standard frontotemporal craniotomy [
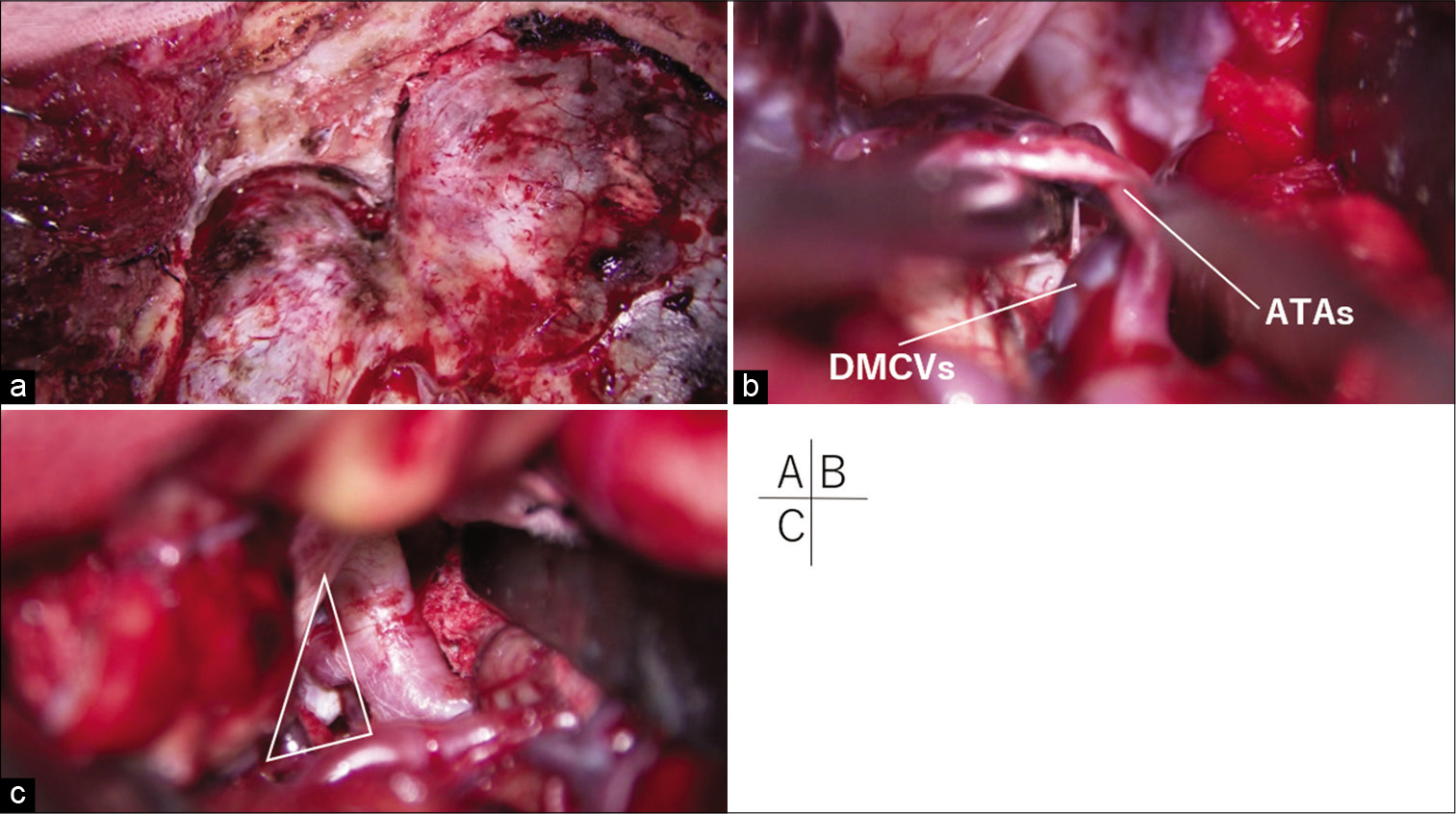
Figure 4:
(a) The superior-lateral wall of the orbit was flattened to the depth of the meningo-orbital band. (b) Dissecting the deep middle cerebral veins (DMCVs) and anterior temporal arteries (ATAs) from the temporal surface enabled gentle posterior retraction of the temporal pole through the space under these vessels. (c) The aneurysm was clipped through widened retrocarotid space (white triangle).
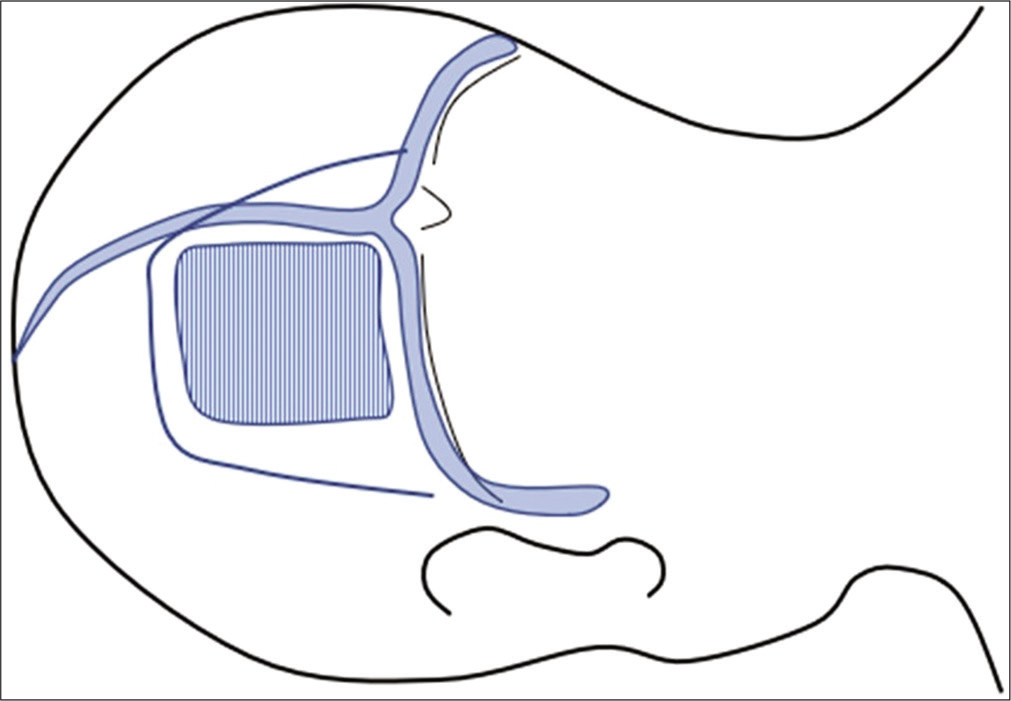
The patient was positioned in left-sided lateral recumbency, and a left rectangular occipital craniotomy along the venous angle made by the superior sagittal sinus and transverse sinus was made [
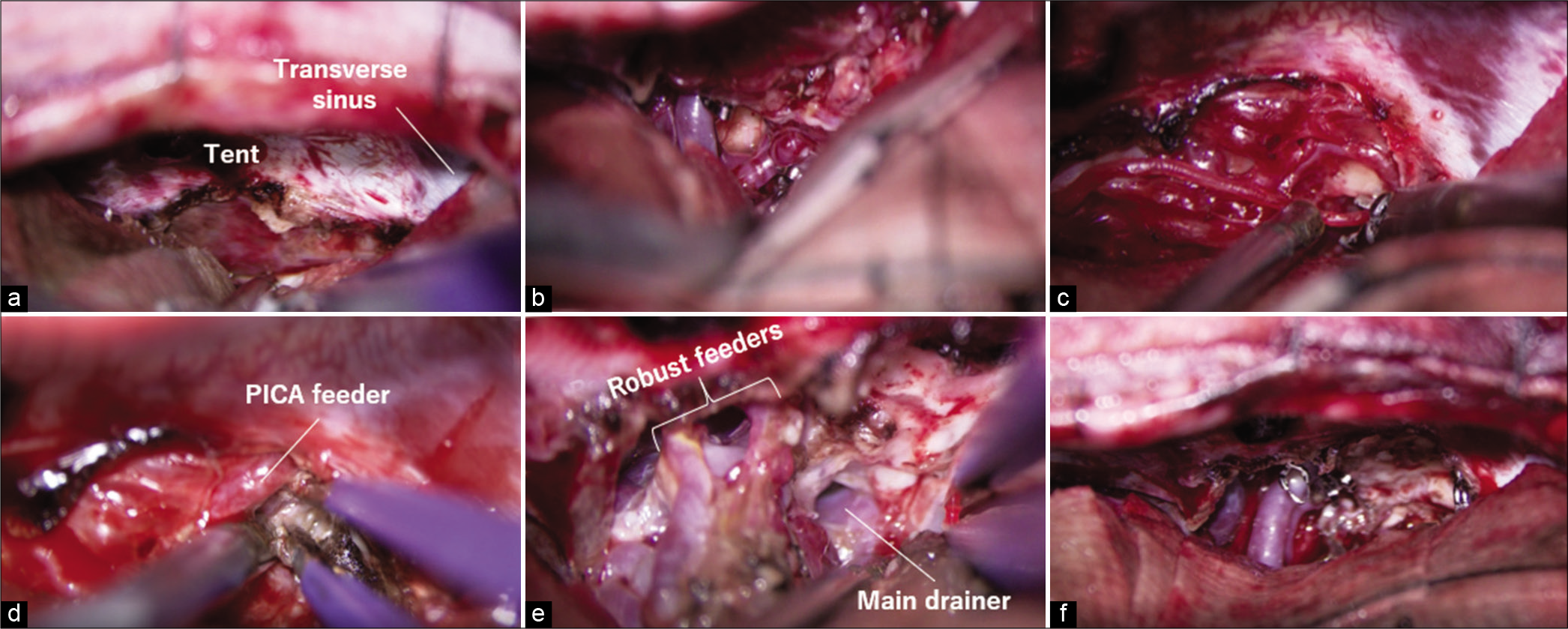
Figure 7:
(a) Tentorial incision parallel to the straight sinus was advanced posteriorly. (b) The quadrigeminal cistern was opened and the main feeders of superior cerebellar artery were clipped preliminary. (c) Relatively thick feeders were separated after reducing the arterial pressure using microclips. (d) The posterior inferior cerebellar artery (PICA) feeder was skeletonized. (e) Robust feeders were fully skeletonized, coagulated, and cut while preserving the passing arteries. (f) The nidus was removed.
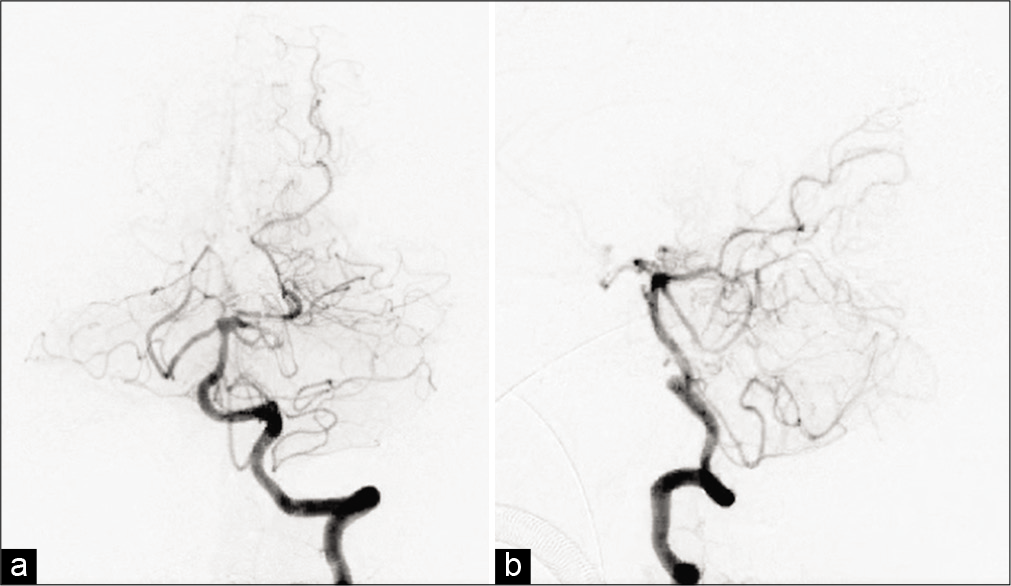
Postoperatively, the pressure in the ventricular drain promptly decreased to <50 mm H2O, and the patient recovered, with improved consciousness to GCS E4V5M6. There were no conspicuous brain infarctions and contusions on postoperative imaging, and the aneurysm and the nidus were completely disappeared in postoperative DSA [
You can see the actual surgical video on electronic supplementary material [
Video 1
Video 2
DISCUSSION
The bleeding frequency in cases of AVM coexisting with an aneurysm is higher than that of AVM alone (1.7% vs. 7% per year, respectively),[
In cases of AVM coexisting with an aneurysm, strategies emphasizing aneurysmal treatment tend to be adopted first considering that if the AVM is treated first, the increased hemodynamic stress to the flow-related aneurysm may result in rupture.
Redekop et al. subdivided flow-related aneurysms into proximal feeder aneurysms existing in the proximal end of the feeding artery (circle of Willis, middle cerebral artery, and vertebrobasilar artery, etc.) and distal feeder aneurysms existing on the trunk of the feeding artery. However, the authors reported that the former has a very low probability of disappearing after AVM treatment (4%), while the latter is likely to regress after AVM treatment (80%).[
Regarding treating the AVM, ruptured aneurysms have a higher risk of re-rupture and worsening.[
In our case, we could not confirm aneurysmal rupture during the initial surgery, and the disorder of the venous return due to the increased venous pressure in the straight sinus associated with the AVM caused increased intracranial pressure; thus, the existence of the AVM was a vital hindrance to managing the SAH. Therefore, we determined that it was desirable to treat the AVM as soon as possible.
Regarding the surgical approach to each lesion, it is generally desirable to treat through a single approach that enables observing both lesions in the same surgical field. To treat the aneurysm, we considered a pterional approach and a subtemporal transtentorial approach, and only the latter allowed access to both lesions. However, in aneurysmal clipping using the subtemporal transtentorial approach, there was a risk of obstruction to the vein of Labbe because of retraction of the temporal lobe of the dominant hemisphere.
As for the AVM, the subtemporal transtentorial approach was suitable for early processing of the ipsilateral SCA, but it was a little too proximal and lateral to expose the contralateral and dorsal surfaces of the AVM and was inappropriate as an approach to the lesion located in the retrocollicular midline region.[
Regarding the approach to the AVM, we considered the suboccipital supracerebellar infratentorial approach and the posterior interhemispheric occipital transtentorial approach (OTA). The AVM in the current case was fed by multiple SCA branches in the precerebellar or quadrigeminal cistern in front of the anterior surface of the cerebellum, and early processing of these arteries was essential to decrease intranidal pressure. However, this space might be hidden by the dilated superior vermian vein or AVM nidus itself in the supracerebellar approach. Furthermore, the increased intranidal pressure and impaired perfusion of the draining vein due to the downward retraction of the swollen cerebellum increased the risk of causing intraoperative AVM rupture;[
Although there are various opinions regarding the patient’s position, we prefer to have the affected sided down, with the patient in lateral recumbency, to allow natural and gentle retraction of the occipital lobe under gravity and to prevent postoperative occipital lobe contusion causing hemianopsia.[
CONCLUSION
We reported an excellent outcome in a case of superior vermian AVM coexisting with a BA-SCA proximal feeder aneurysm successfully treated by a two-stage operation in the acute phase of SAH.
In particular, disorders of intracranial venous return associated with an AVM can be a vital hindrance to managing cerebral vasospasm, and treating both lesions in the acute phase may lead to good outcomes.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos available on:
www.surgicalneurologyint.com
References
1. Aoki N. Combined occipital transtentorial and infratentorial supracerebellar approach in the concorde position for the treatment of an arteriovenous malformation in the upper vermis: Case report. Neurosurgery. 1985. 17: 815-7
2. Ausman JI, Malik GM, Dujovny M, Mann R. Three-quarter prone approach to the pineal-tentorial region. Surg Neurol. 1988. 29: 298-306
3. Brotchi J, Levivier M, Raftopoulos C, Dewitte O, Pirotte B, Noterman J, Koos W, Richling B.editors. Three-quarter prone approach to the pinealtentorial region, Report of seven cases. Processes of the Cranial Midline, Acta Neurochirurgica Supplementum. Vienna: Springer; 1991. p. 144-7
4. Brown RD, Wiebers DO, Forbes GS. Unruptured intracranial aneurysms and arteriovenous malformations: Frequency of intracranial hemorrhage and relationship of lesions. J Neurosurg. 1990. 73: 859-63
5. da Costa L, Thines L, Dehdashti AR, Wallace MC, Willinsky RA, Tymianski M. Management and clinical outcome of posterior fossa arteriovenous malformations: Report on a single-centre 15-year experience. J Neurol Neurosurg Psychiatry. 2009. 80: 376-9
6. Graf CJ, Perret GE, Torner JC. Bleeding from cerebral arteriovenous malformations as part of their natural history. J Neurosurg. 1983. 58: 331-7
7. Jafar JJ, Rezai AR. Acute surgical management of intracranial arteriovenous malformations. Neurosurgery. 1994. 34: 8-13
8. Kassell NF, Torner JC. Aneurysmal rebleeding: A preliminary report from the cooperative aneurysm study. Neurosurgery. 1983. 13: 479-81
9. Lawton MT, Du R, Tran MN, Achrol AS, McCulloch CE, Johnston SC. Effect of presenting hemorrhage on outcome after microsurgical resection of brain arteriovenous malformations. Neurosurgery. 2005. 56: 485-93
10. Liu HM, Wang YH, Chen YF, Tu YK, Huang KM. Endovascular treatment of brain-stem arteriovenous malformations: Safety and efficacy. Neuroradiology. 2003. 45: 644-9
11. McLaughlin N, Martin NA. The occipital interhemispheric transtentorial approach for superior vermian, superomedian cerebellar, and tectal arteriovenous malformations: advantages, limitations, and alternatives. World Neurosurg. 2014. 82: 409-16
12. Ondra SL, Troupp H, George ED, Schwab K. The natural history of symptomatic arteriovenous malformations of the brain: A 24-year follow-up assessment. J Neurosurg. 1990. 73: 387-91
13. Pavesi G, Rustemi O, Berlucchi S, Frigo AC, Gerunda V, Scienza R. Acute surgical removal of low-grade (SpetzlerMartin I-II) bleeding arteriovenous malformations. Surg Neurol. 2009. 72: 662-7
14. Redekop G, TerBrugge K, Montanera W, Willinsky R. Arterial aneurysms associated with cerebral arteriovenous malformations: Classification, incidence, and risk of hemorrhage. J Neurosurg. 1998. 89: 539-46
15. Rodríguez-Hernández A, Kim H, Pourmohamad T, Young WL, Lawton MT. Cerebellar arteriovenous malformationsanatomic subtypes, surgical results, and increased predictive accuracy of the supplementary grading system. Neurosurgery. 2012. 71: 1111-24
16. Salcman M, Nudelman RW, Bellis EH. Arteriovenous malformations of the superior cerebellar artery: Excision via an occipital transtentorial approach. Neurosurgery. 1985. 17: 749-56
17. Solomon RA, Stein BM. Surgical management of arteriovenous malformations that follow the tentorial ring. Neurosurgery. 1986. 18: 708-15
18. Stein BM, Solomon RA. Surgical approaches to posterior fossa arteriovenous malformations. Clin Neurosurg. 1991. 37: 353-71
19. Uchino H, Akioka N, Tomita T, Kashiwazaki D, Kuwayama N, Kuroda S. Removal of superior vermian arteriovenous malformation through the occipital transtentorial approach. Interdiscip Neurosurg. 2019. 17: 84-6
20. Uno M, Satoh K, Matsubara S, Satomi J, Nakajima N, Nagahiro S. Does multimodality therapy of arteriovenous malformations improve patient outcome?. Neurol Res. 2004. 26: 50-4