- Department of Neurosurgery, Faculty of Medicine, Universitas Padjadjaran–Dr. Hasan Sadikin Hospital, Bandung, West Java, Indonesia
- Department of Pathology Anatomy, Faculty of Medicine, Universitas Padjadjaran–Dr. Hasan Sadikin Hospital, Bandung, West Java, Indonesia
Correspondence Address:
Ahmad Faried
Department of Pathology Anatomy, Faculty of Medicine, Universitas Padjadjaran–Dr. Hasan Sadikin Hospital, Bandung, West Java, Indonesia
DOI:10.4103/2152-7806.185782
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Faried A, Pribadi MA, Sumargo S, Arifin MZ, Hernowo BS. Adult medulloblastoma: A rare case report and literature review. Surg Neurol Int 07-Jul-2016;7:
How to cite this URL: Faried A, Pribadi MA, Sumargo S, Arifin MZ, Hernowo BS. Adult medulloblastoma: A rare case report and literature review. Surg Neurol Int 07-Jul-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/adult-medulloblastoma-rare-case-report-literature-review/
Abstract
Background:Medulloblastoma is a highly malignant embryonal tumor which commonly arises in the cerebellum. It is relatively rare and accounts for less than 2% of all primary brain tumors. The tumor primarily occurs in childhood; however, rarely, it may be found in adult population. In addition, medulloblastoma in adult population shows features which are quite distinct from the pediatric group.
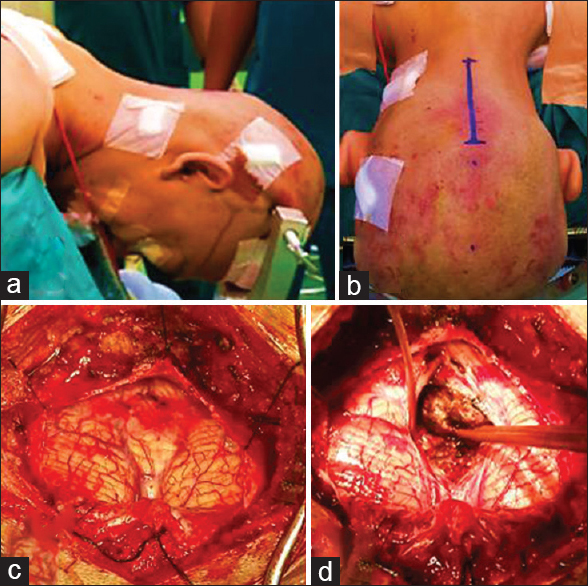
Case Description:We report the case of a 33-year-old man who presented to our institution with a history of blurred vision of both eyes for 5 months preceded by intermittent headache since the previous year. Preoperative investigation suggested a posterior fossa mass and we suspected an ependymoma. The patient underwent ventriculoperitoneal shunt and craniotomy tumor removal, followed by radiotherapy. Histopathological and immunohistochemical examination were performed, and the results showed a diagnosis of medulloblastoma.
Conclusion:This case is exceptional because adult medulloblastoma occurrence in our center is extremely rare, and the diagnosis can only be established through histopathological and immunohistochemical studies.
Keywords: A rare case of adult medulloblastoma, fossa posterior brain tumor, immunohistochemical studies
INTRODUCTION
Medulloblastoma is a malignant embryonal tumor that commonly arises in the cerebellum.[
CASE REPORT
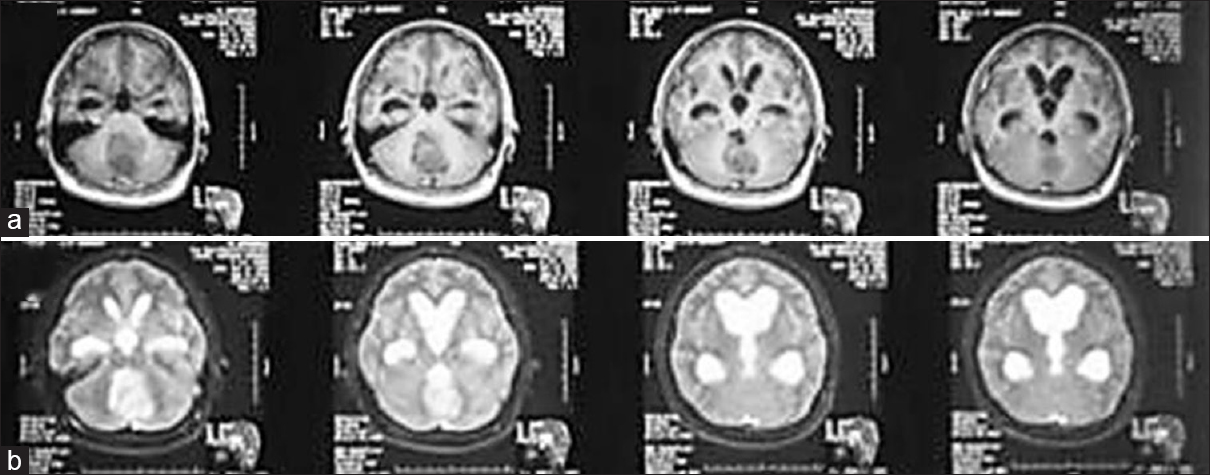
A 33-year-old man came to our center with blurred vision in both eyes for 5 months as the chief complaint. This was also accompanied with intermittent headaches since the previous year. Neurological findings showed that visual acuity in both eyes was 3/60 without papilledema. Other cranial nerves were within normal limit, and there was no motor or sensory deficit. Magnetic resonance imaging (MRI) of the head was conducted that showed a midline posterior fossa mass, which had an iso-hypointense appearance on T1-weighted images, an inhomogenously enhanced appearance in T1-weighted contrast images, and iso-hyperintensity in T2-weighted images. The MRI also revealed that the temporal horns were more than 2 mm with frontal horns-internal diameter ratio (FH/ID) of 50% and the Evan ratio of 0.3. [Figure
Figure 4
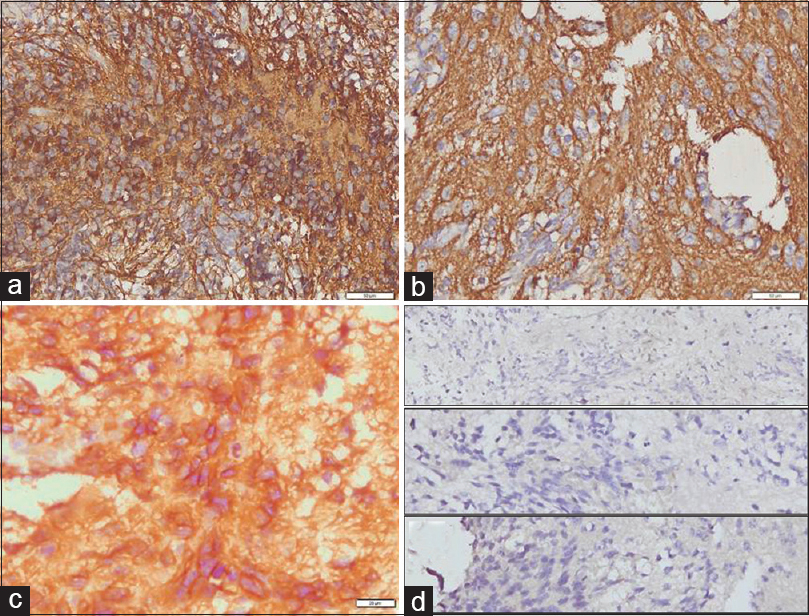
Immunohistochemistry analysis was conducted and the result showed that the tumor is positive for glial fibrillary acidic protein, synaptophysin, and smooth muscle actin (a-c), whereas negative for cytokeratin, desmin, and epithelial membrane antigen (d, from upper, middle, and lower figures, respectively)
DISCUSSION
Medulloblastoma is a highly malignant tumor of the central nervous system which commonly arises from the cerebellar vermis in the roof of fourth ventricle.[
Patients with medulloblastoma classically present to medical facilities with clinical signs and symptoms of increased intracranial pressure due to cerebrospinal fluid flow obstruction or cerebellar dysfunction. Headache, nausea, vomiting, and blurred vision are the most common presenting manifestations. Funduscopic examination shows papilledema. As the disease progresses and the tumor infiltrates the brainstem, cranial nerves dysfunction becomes more common. Nystagmus and diplopia manifests as the results of cranial nerve IV or VI palsy. Ataxia is also commonly seen.[
Computed tomography (CT) and MRI of medulloblastoma is quite distinct between adults and children. In children, the typical medulloblastoma has been well-described in CT imaging. The tumor in pediatric groups appears as a well-defined, homogeneous, hyperdense, median mass in CT contrast imaging. Medulloblastoma in adults rarely gives this appearance but shows more variable findings, that is, poorly defined, less enhanced with contrast, inhomogeneous due to cystic, necrotic degeneration, and more laterally placed.[
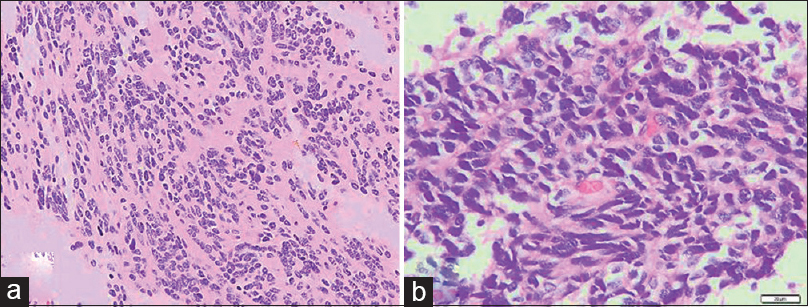
Histopathologically, classic medulloblastoma is extremely cellular with sheets of anaplastic cells and abundant mitosis, whereas the individual cells are small, with scant cytoplasm and hyperchromatic nuclei which are frequently elongated or crescent shaped. The tumor may express neurosecretory granules or Homer Wright rosettes.[
CONCLUSION
Medulloblastoma is particularly rare in adults and the diagnosis is difficult to be established. The imaging modalities alone cannot determine the diagnosis of medulloblastoma in adults, given that the tumor shows a wide range of features which can be confused with other posterior fossa masses. In our case, the diagnosis of medulloblastoma can only be confirmed by means of histopathological and immunohistochemical examination.
Consent
Informed consent was obtained from the patient for publication of this case report and any accompaning images. His family was present at the time.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AF, MAP, SS, and MZ had examined, treated, observed, and followed-up the participant of this research. AP and AF performed the operation on the patient. BSH carried out the histopathological studies and intepreted the results of the patient's tissue. All authors participated in writing the manuscript. All authors have read and approved the final manuscript.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors would like to thank Regina Melia from the Department of Neurosurgery, Faculty of Medicine, Universitas Padjadjaran–Dr. Hasan Sadikin Hospital, for her technical assistants.
References
1. .editors. American Brain Tumor Association. Medulloblastoma. Association ABT. Chicago: 2012. p.
2. Bourgouin PM, Tampieri D, Grahovac SZ, Léger C, Carpio RD, Melançon D. CT and MR imaging findings in adults with cerebellar medulloblastoma: Comparison with findings in children. Am J Roentgenol. 1992. 159: 609-12
3. Brandes AA, Franceschi E, Tosoni A, Reni M, Gatta G, Vecht C. Adult neuroectodermal tumors of posterior fossa (medulloblastoma) and of supratentorial sites (stPNET). Crit Rev Oncol Hematol. 2009. 71: 165-79
4. Brandes AA, Paris MK. Review of the prognostic factors in medulloblastoma of children and adults. Crit Rev Oncol Hematol. 2004. 50: 121-8
5. Ellison DW, Dalton J, Kocak M, Nicholson SL, Fraga C, Neale G. Medulloblastoma: Clinico-pathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathol. 2011. 121: 381-96
6. Farwell J, Flannery J. Adult occurrence of medulloblastoma. Acta Neurochir. 1987. 86: 1-5
7. Frosch MP, Anthony DC, de Girolami U, Kumar V, Abbas AK, Fausto N, Aster JC.editors. The central nervous system. Robbins Cotran Pathologic Basis of Disease. Philadelphia: Saunders Elsevier; 2010. p.
8. Giordana MT, Cavalla P, Dutto A, Borsotti L, Chiò A, Schiffer D. Is medulloblastoma the same tumor in children and adults?. J Neuro-Oncol. 1997. 35: 169-76
9. Giordana MT, Schiffer P, Lanotte M, Girardi P, Chio A. Epidemiology of adult medulloblastoma. Int J Cancer. 1999. 80: 689-92
10. Ho DM, Hsu CY, Chiang H. Histopathologic grading of medulloblastomas. Cancer. 2002. 95: 2577-9
11. Kool M, Korshunov A, Remke M, Jones DTW, Schlanstein M, Northcott PA. Molecular subgroups of medulloblastoma: An international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012. 123: 473-84
12. Malheiros SMF, Carrete JH, Stávale JN, Santos AJ, Borges LRR, Guimarães IF. MRI of medulloblastoma in adults. Neuroradiology. 2003. 45: 463-7
13. Marino S, Vooijs M, Gulden Hvd, Jonkers J, Berns A. Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes Dev. 2000. 14: 994-1004
14. Neto AdC, Gasparetto EL, Ono SE, Bertoldi GA, Gomes AF. Adult cerebellar medulloblastoma CT and MRI findings in eight cases. Arq Neuropsiquiatr. 2003. 61: 199-203
15. Packer RJ, Cogen P, Vezina G, Rorke LB. Medulloblastoma: Clinical and biologic aspects. Neuro Oncol. 1999. 1: 232-50
16. Remke M, Hielscher T, Northcott PA, Witt H, Ryzhova M, Wittmann A. Adult medulloblastoma comprises three major molecular variants. J Clin Oncol. 2011. 29: 2717-23
17. Rooper AH, Brown RH.editorsIntracranial neoplasms and paraneoplastic disorders. Adams and Victor's Principles of Neurology. New York: McGraw-Hill; 2005. p.
18. Schüller U, Heine VM, Mao J, Kho AT, Dillon AK, Han YG. Acquisition of granule neuron precursor identity is a critical determinant of progenitor cell competence to form Hedgehog induced medulloblastoma. Cancer Cell. 2008. 12: 123-34
19. Smoll NR, Drummond KJ. The incidence of medulloblastomas and primitive neurectodermal tumours in adults and children. J Clin Neurosci. 2012. 19: 1541-4
20. Spassky N, Han Y-G, Aguilar A, Strehl L, Besse L, Laclef C. Primary cilia are required for cerebellar development and Shh-dependent expansion of progenitor pool. Dev Biol. 2008. 317: 246-59
21. Sutter R, Shakhova O, Bhagat H, Behesti H, Sutter C, Penkar S. Cerebellar stem cells act as medulloblastoma-initiating cells in a mouse model and a neural stem cell signature characterizes a subset of human medulloblastomas. Oncogene. 2010. 29: 1845-56
22. Takei H, Bhattacharjee MB, Rivera A, Dancer Y, Powell SZ. New immunohistochemical markers in the evaluation of central nervous system tumors: A review of 7 selected adult and pediatric brain tumors. Arch Pathol Lab Med. 2007. 131: 234-41
23. Takei H, Powell SZ, Hayat MA.editors. Diagnosing and grading of brain tumor: Immunohistochemistry. Methods of cancer diagnosis, therapy, and prognosis: Brain cancer. Netherlands: Springer; 2010. p. 43-
24. Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC. Molecular subgroups of medulloblastoma: The current consensus. Acta Neuropathol. 2012. 123: 465-72
25. Tortori-Donati P, Fondelli MP, A. Rossi AC, Caputo L, Andreussi L, Garré ML. Medulloblastoma in children: CT and MRI findings. Neuroradiology. 1996. 38: 352-9
26. Yang ZJ, Ellis T, Markant SL, Read TA, Kessler JD, Bourboulas M. Medulloblastoma can be initiated by deletion of patched in lineage-restricted progenitors or stem cells. Cancer Cell. 2008. 14: 135-45
27. Zhang J, Jiao J. Molecular biomarkers for embryonic and adult neural stem cell and neurogenesis. BioMed Res Int. 2015. p.