- Departments of Neurosurgery and Neurology, IRCCS Istituto Ortopedico Galeazzi, Milan, Italy
Correspondence Address:
Domenico Servello
Departments of Neurosurgery and Neurology, IRCCS Istituto Ortopedico Galeazzi, Milan, Italy
DOI:10.4103/sni.sni_271_18
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Servello D, Saleh C, Bona AR, Zekaj E, Porta M. After 19 years of deep brain stimulation in Tourette's syndrome: From multiple targets to one single target?. Surg Neurol Int 30-Oct-2018;9:219
How to cite this URL: Servello D, Saleh C, Bona AR, Zekaj E, Porta M. After 19 years of deep brain stimulation in Tourette's syndrome: From multiple targets to one single target?. Surg Neurol Int 30-Oct-2018;9:219. Available from: http://surgicalneurologyint.com/surgicalint-articles/9056/
The medical scientific community drives toward standardization of procedure. In deep brain stimulation (DBS) for motor diseases, there are two main targets; the subthalamic nucleus and the globus pallidus internus (GPi). In contrast, in DBS for Tourette's syndrome (TS), currently there is no consensus as to target choice. Multiple potential targets are proposed and are used, furthermore in single or combined fashion. Is a single target for DBS in TS ultimately possible? DBS for medication refractory TS was proposed in 1999 by the Dutch team led by Vandewalle[
To address such a variable range of symptoms and reduce social impairment, clinicians have progressively shifted their attention from a simple treatment of tics to a wider control of comorbidities. During the last two decades, beside the CM/Pf of the thalamus, new DBS targets have been investigated for a better control of comorbidities. Currently, there is the tendency to select targets based on the specific phenotypic presentation,[
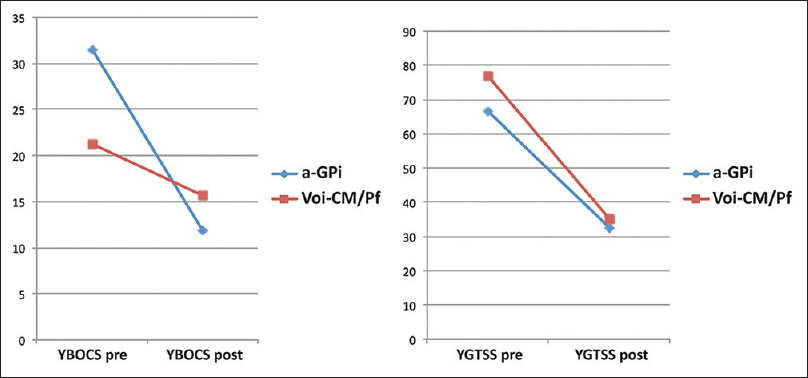
Currently, based on double-blind studies, the most robust data come from thalamic and pallidal stimulation [
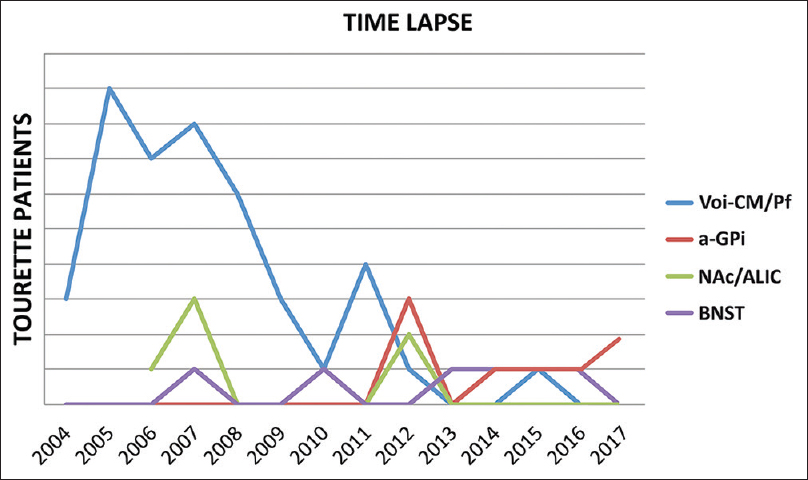
To the best of our knowledge, we have worldwide the largest data pool on DBS in TS. We performed 66 DBS procedures on 61 TS patients between 2004 and 2017. From 2004 to 2012, we treated 42 TS patients targeting the ventro-oralis-internus centromedian parafascicular thalamus (Voi-CM/Pf), which is located 2 mm anteriorly to the target described by Vandewalle. Since the beginning, we decided to locate the DBS-lead anteriorly for a better stimulation of the associative-limbic connections, in order to modulate both motors as behavioral features of TS.[
Figure 2
Procedure targeting evolution from 2004 to 2017. The ventro-oralis-internus centromedian parafascicular thalamus (Voi-CM/Pf) depicted in blue, the nucleus accumbens/anterior limb of the internal capsule (NAc/ALIC) depicted in green, the bed nucleus of stria terminalis (BNST) depicted in purple, and the antero-medial globus pallidus internus (a-GPi) depicted in red
On a concluding note:
The current most critical aspect in DBS TS remains the standardization of target choice. Our center has to the best of our knowledge the worldwide largest data pool on DBS in TS. Based on our experience and from the published data, it appears that a single target in TS is possible. We think that the limbic a-GPi is a promising target in pharmacologic refractory TS for motor and for limbic symptoms. With our short communication, we hope to drive the DBS community to pay more attention to this target to achieve a consensus on target selection in TS. Consensus on target selection would aid significantly in standardizing further the procedures in DBS for TS, in order to improve patient care.
References
1. Ackermans L, Duits A, van der Linden C, Tijssen M, Schruers K, Temel Y. Double-blind clinical trial of thalamic stimulation in patients with Tourette syndrome. Brain. 2011. 134: 832-44
2. Dell’Osso B, Marazziti D, Albert U, Pallanti S, Gambini O, Tundo A. Parsing the phenotype of obsessive-compulsive tic disorder (OCTD): A multidisciplinary consensus. Int J Psychiatry Clin Pract. 2017. 21: 156-9
3. Gomes de Alvarenga P, de Mathis MA, Dominguez Alves AC, do Rosário MC, Fossaluza V, Hounie AG. Clinical features of tic-related obsessive-compulsive disorder: Results from a large multicenter study. CNS Spectr. 2012. 17: 87-93
4. Hassler R, Dieckmann G. Stereotaxic treatment of tics and inarticulate cries or coprolalia considered as motor obsessional phenomena in Gilles de la Tourette's disease. Rev Neurol (Paris). 1970. 123: 89-100
5. Huasen B, McCreary R, Evans J, Potter G, Silverdale M. Cervical myelopathy secondary to Tourette's syndrome managed by urgent deep brain stimulation. Mov Disord. 2014. 29: 452-3
6. Kefalopoulou Z, Zrinzo L, Jahanshahi M, Candelario J, Milabo C, Beigi M. Bilateral globus pallidus stimulation for severe Tourette's syndrome: A double-blind, randomised crossover trial. Lancet Neurol. 2015. 14: 595-605
7. Martínez-Fernández R, Zrinzo L, Aviles-Olmos I, Hariz M, Martinez-Torres I, Joyce E. Deep brain stimulation for Gilles de la Tourette syndrome: A case series targeting subregions of the globus pallidus internus. Mov Disord. 2011. 26: 1922-30
8. Martinez-Ramirez D, Jimenez-Shahed J, Leckman JF, Porta M, Servello D, Meng FG. Efficacy and safety of deep brain stimulation in Tourette syndrome: The international Tourette syndrome deep brain stimulation public database and registry. JAMA Neurol. 2018. 75: 353-9
9. Massano J, Sousa C, Foltynie T, Zrinzo L, Hariz M, Vaz R. Successful pallidal deep brain stimulation in 15-year-old with Tourette syndrome: 2-year follow-up. J Neurol. 2013. 260: 2417-9
10. Porta M, Saleh C, Zekaj E, Zanaboni Dina C, Bona AR, Servello D. Why so many deep brain stimulation targets in Tourette's syndrome. Toward a broadening of the definition of the syndrome?. J Neural Transm (Vienna). 2016. 123: 785-90
11. Porta M, Sassi M, Ali F, Cavanna AE, Servello D. Neurosurgical treatment for Gilles de la Tourette syndrome: The Italian perspective. J Psychosom Res. 2009. 67: 585-90
12. Robertson MM, Cavanna AE, Eapen V. Gilles de la Tourette syndrome and disruptive behavior disorders: Prevalence, associations, and explanation of the relationships. J Neuropsychiatry Clin Neurosci. 2015. 27: 33-41
13. Sachdev PS, Mohan A, Cannon E, Crawford JD, Silberstein P, Cook R. Deep brain stimulation of the antero-medial globus pallidus interna for Tourette syndrome. PLoS One. 2014. 9: e104926-
14. Servello D, Sassi M, Brambilla A, Porta M, Haq I, Foote KD. De novo and rescue DBS leads for refractory Tourette syndrome patients with severe comorbid OCD: A multiple case report. J Neurol. 2009. 256: 1533-9
15. Servello D, Zekaj E, Saleh C, Lange N, Porta M. Deep brain stimulation in Gilles de la Tourette syndrome: What does the future hold? A Cohort of 48 Patients. Neurosurgery. 2016. 78: 91-100
16. Vandewalle V, van der Linden C, Groenewegen HJ, Caemaert J. Stereotactic treatment of Gilles de la Tourette syndrome by high frequency stimulation of thalamus. Lancet. 1999. 353: 724-
17. Welter M-L, Houeto J-L, Thobois S, Bataille B, Guenot M, Worbe Y. Anterior pallidal deep brain stimulation for Tourette's syndrome: A randomised, double-blind, controlled trial. Lancet Neurol. 2017. 16: 610-9
18. Zapparoli L, Porta M, Gandola M, Invernizzi P, Colajanni V, Servello D. A functional magnetic resonance imaging investigation of motor control in Gilles de la Tourette syndrome during imagined and executed movements. Eur J Neurosci. 2016. 43: 494-508
19. Zapparoli L, Porta M, Paulesu E. The anarchic brain in action: The contribution of task-based fMRI studies to the understanding of Gilles de la Tourette syndrome. Curr Opin Neurol. 2015. 28: 604-11