- Department of Neurosurgery, Nova Lima, Minas Gerais, Brazil,
- Pathology, Biocor Instituto, Nova Lima, Minas Gerais, Brazil,
- Department of Neurosurgery, Instituto de Assistência Médica ao Servidor Público do Estado de São Paulo (IAMSPE), São Paulo, São Paulo, Brazil.
Correspondence Address:
François Dantas, Department of Neurosurgery, Biocor Instituto, Nova Lima, Minas Gerais, Brazil.
DOI:10.25259/SNI_304_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: François Dantas1, Jair Leopoldo Raso1, Patrícia Salomé Gouvea Braga2, Ricardo Vieira Botelho3, Fernando Luiz Rolemberg Dantas1. Aggressive dissemination of central nervous system hemangioblastoma without association with von Hippel–Lindau disease: A case report and literature review. 12-Aug-2022;13:358
How to cite this URL: François Dantas1, Jair Leopoldo Raso1, Patrícia Salomé Gouvea Braga2, Ricardo Vieira Botelho3, Fernando Luiz Rolemberg Dantas1. Aggressive dissemination of central nervous system hemangioblastoma without association with von Hippel–Lindau disease: A case report and literature review. 12-Aug-2022;13:358. Available from: https://surgicalneurologyint.com/surgicalint-articles/11787/
Abstract
Background: Hemangioblastomas (HBs) typically present with benign behavior and are most commonly found in the posterior fossa. Multiple central nervous system (CNS) HBs are usually associated with von Hippel–Lindau disease, and leptomeningeal dissemination of sporadic HBs is extremely rare. A review of the literature identified 30 cases of leptomeningeal dissemination of sporadic HBs previously published in the literature.
Case Description: We report the case of a patient who was diagnosed with multiple CNS HBs with aggressive progression 6 years after resection of a posterior fossa HB. He underwent multiple surgeries and died 4 years after the diagnosis of the first spinal dissemination.
Conclusion: Dissemination of sporadic HBs is rare and aggressive disease evolution is usually observed. Further studies are necessary to determine the optimal therapeutic options.
Keywords: Case report, Central nervous system, Hemangioblastoma, Hemangioblastomatosis, von Hippel–Lindau
INTRODUCTION
Hemangioblastomas (HBs) account for approximately 2% of central nervous system (CNS) tumors, usually have benign characteristics, and are well-circumscribed and highly vascular neoplasms. They account for 7.5% and 5% of all adult tumors of the posterior fossa and spinal cord, respectively.[
Approximately 66% of CNS HBs occur sporadically, and the remainder occurs in the context of von Hippel–Lindau (VHL) disease, an autosomal dominant neoplasia syndrome caused by a mutation in the VHL tumor suppressor gene.[
Although the dissemination of HBs in the CNS can occur frequently in patients with VHL disease, it is a rare condition in cases of sporadic HBs, and few cases have been reported in the literature. Herein, we report a case of aggressive craniospinal dissemination of sporadic HB in a previously healthy patient who underwent multiple surgeries and died 4 years after the diagnosis of the first spinal dissemination due to disease progression. We conducted a literature review to identify similar cases and treatment options.
CASE REPORT
A 37-year-old man with no previous medical conditions was diagnosed with a posterior fossa tumor in 2011 and underwent surgery at another institution. Histopathological analysis was consistent with HB. The patient developed recurrence of the cerebellar lesion and underwent a new surgical approach and neoadjuvant stereotactic radiosurgery in 2014. In 2017, he was diagnosed with a lesion at the Th5 level and underwent partial resection of the lesion and subsequent stereotactic radiosurgery. Detailed records of the patient’s previous procedures and treatments are not available.
In July 2019, the patient was referred to our neurosurgery facility; he was 45 years of age at the time. On admission, the patient presented with persistent neuropathic pain in the Th6 dermatome, radicular pain in the L1 dermatome, and mild paraparesis. Neuraxial magnetic resonance imaging (MRI) showed multiple contrast-enhancing brain and spinal cord lesions, with leptomeningeal enhancement [
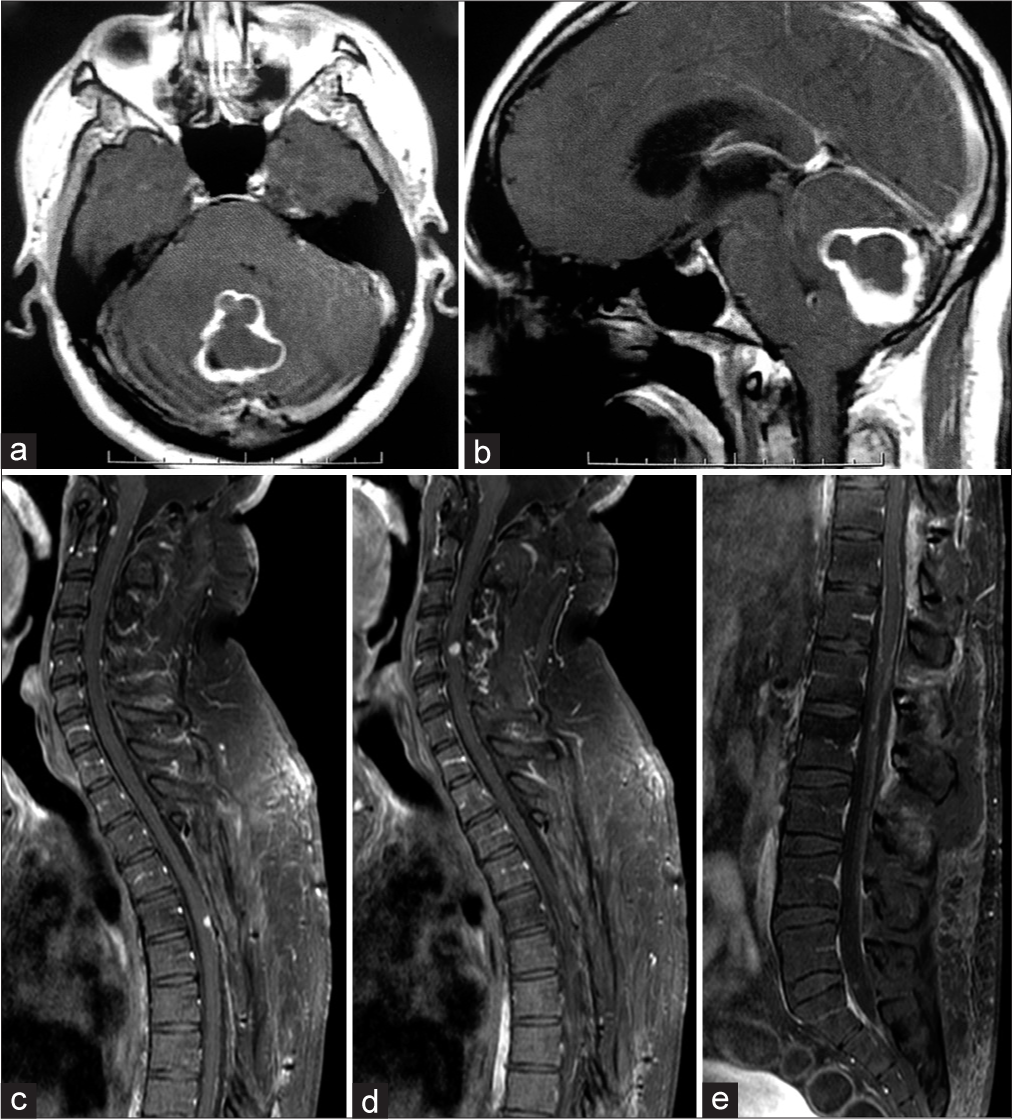
Figure 1:
Gadolinium-enhanced T1-weighted axial (a) and sagittal (b) MRI showing a midline lesion in the posterior fossa with peripheral contrast enhancement in January 2011. Gadolinium-enhanced sagittal T1-weighted MRI showing multiple intradural extramedullary spinal lesions at C1, Th5 (c), and C5 (d), with leptomeningeal enhancement (e) in July 2019.
In July 2019, the patient underwent gross total resection (GTR) of intradural and extramedullary lesions at Th10 and L1, both with histopathological analysis compatible with HB [
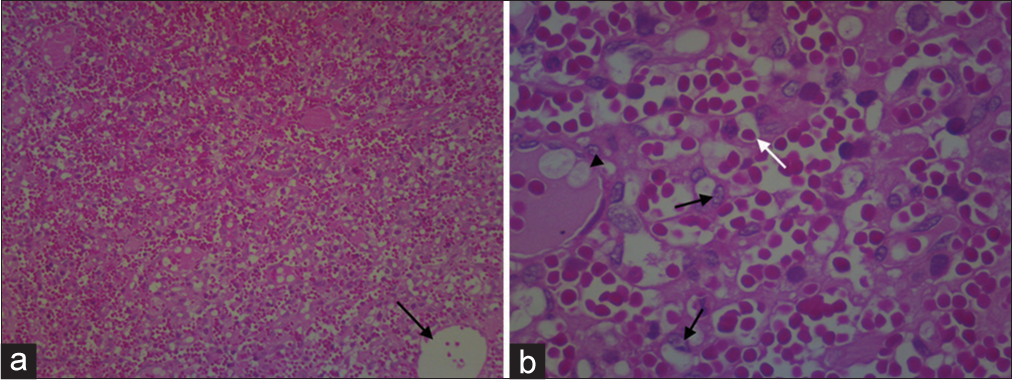
Figure 2:
Photomicrograph (H&E, ×40) showing a diffuse and monomorphic pattern of clear cell proliferation amidst a rich vascular network; the black arrow indicates a vessel with an increased caliber in relation to most vessels, which have a capillary caliber (a). Photomicrograph (H&E, ×400) showing detail of clear cell proliferation without atypia (black arrows) amid numerous capillary blood vessels (white arrow) and major vessels (black arrowhead) (b).
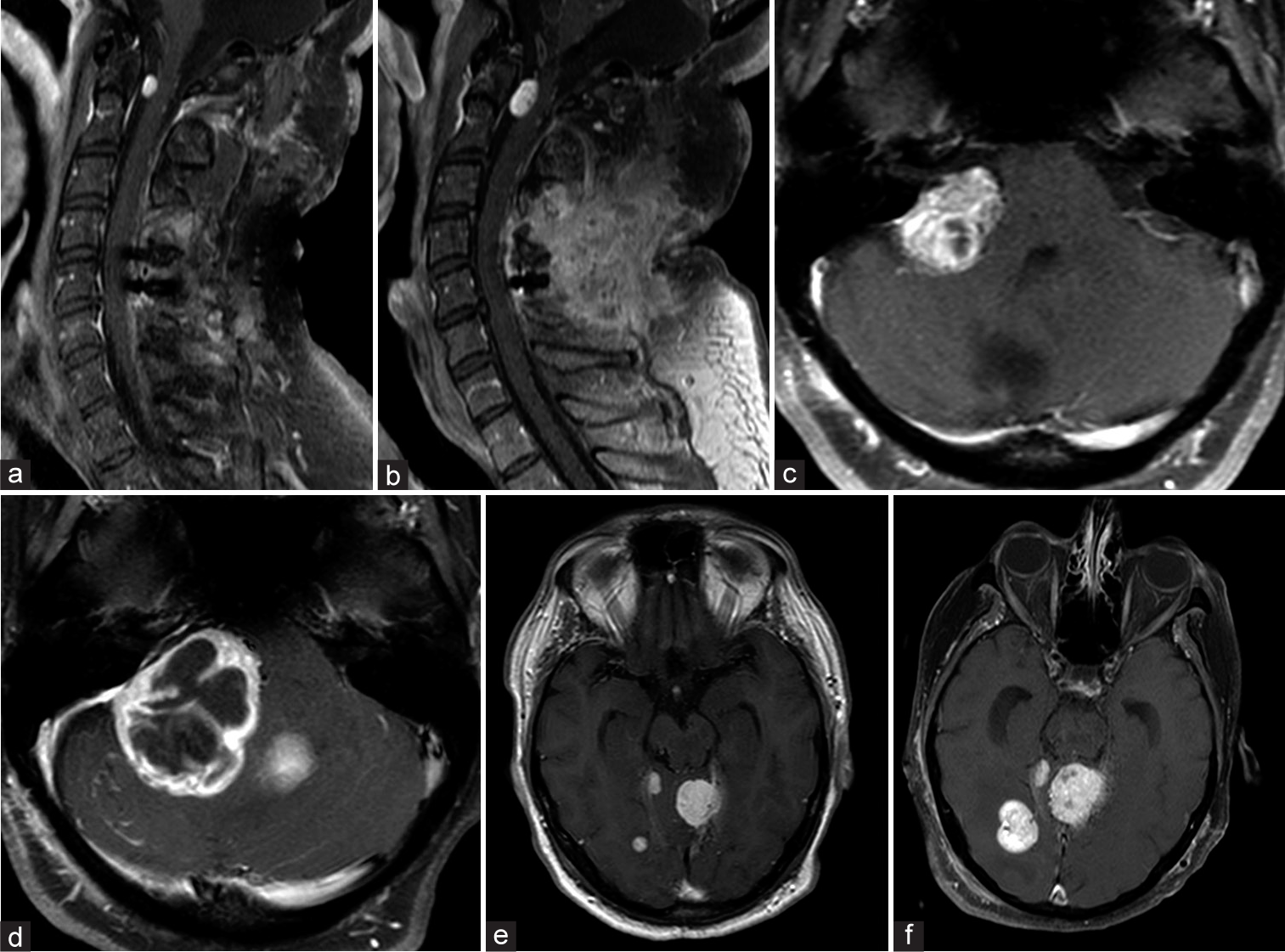
Figure 3:
Images demonstrating the aggressive progression of the lesions. Gadolinium-enhanced T1-weighted sagittal cervical spine MRI showing a ventral intradural extramedullary lesion at C1 in October 2019 (a); MRI performed in December 2019 demonstrated rapid progression of the lesion and spinal cord compression (b). MRI showing a right cerebellopontine angle tumor in December 2019 (c); expansion of the lesion was observed in April 2020, causing brainstem compression and fourth ventricle displacement (d). MRI showing a small right temporo-occipital tumor and a left tentorial tumor in January 2020 (e); growth of both lesions was observed in July 2020 (f).
In January 2020, he underwent GTR of a C1 lesion and a small median cerebellar lesion, when he developed worsening quadriparesis in the immediate postoperative period and CSF leak, which was surgically corrected. In March 2020, he presented with a persistent headache and underwent endoscopic third ventriculostomy for the treatment of hydrocephalus.
He developed vertigo, dysphagia, and right hearing loss in April 2020 when he was hospitalized again for complete resection of a large lesion in the right cerebellopontine angle. The patient developed postoperative worsening of dysphagia and CNS infection, which was treated with antibiotic therapy. At this time, two genetic tests for VHL gene mutations in blood DNA were performed, both of which were negative. There was no family history of VHL or stigmas suggesting the presence of the disease in other family members.
In June 2020, he underwent ventriculoperitoneal shunt, when he already presented with bradypsychism and
The patient developed a generalized tonic-clonic seizure at home in early March 2021, which progressed to aspiration pneumonia. He was readmitted for the treatment of the infectious condition, but due to the prior neurological condition and clinical severity, palliative care was chosen together with the family. The patient died in March 2021 at the age of 47 years, 10 years after the first surgery, 7 years after the first local recurrence, and 4 years after the diagnosis of spinal dissemination.
DISCUSSION
HBs are highly vascularized tumors, most commonly located in the posterior fossa, and typically have benign characteristics, accounting for approximately 2% of CNS tumors and 5% of spinal cord tumors.[
VHL is an autosomal dominant syndrome caused by a mutation in the VHL tumor suppressor gene, with a prevalence of 1:36,000 births. Patients with VHL may develop visceral lesions such as renal cell carcinomas or cysts, pheochromocytomas, and pancreatic neuroendocrine tumors. CNS lesions include retinal and neuroaxis HB as well as endolymphatic sac tumors.[
There are several case reports and case series in the literature regarding multiple CNS HBs in patients with VHL;[
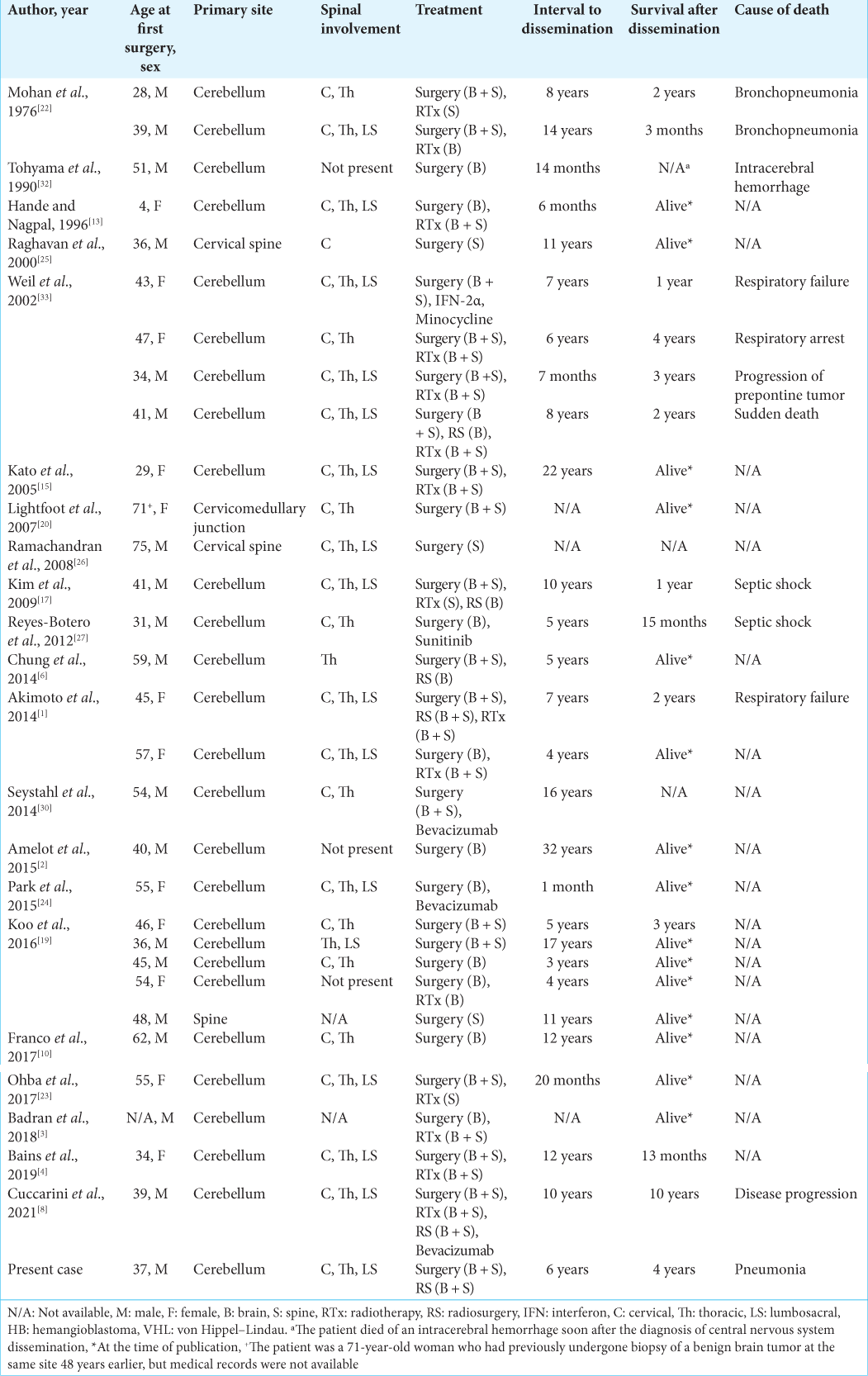
After an extensive review of the literature in PubMed and Google Scholar using the terms “Hemangioblastoma,” “Hemangioblastomatosis,” “Leptomeningeal dissemination,” and “von Hippel–Lindau,” we found 30 similar cases reported in the past 46 years [
Patient characteristics, treatments, and survival time after the initial diagnosis of CNS dissemination varied among previously reported studies. Surgery was the mainstay treatment in most cases, and adjuvant therapies were variable, including radiotherapy, radiosurgery, and chemotherapy.
The term hemangioblastomatosis is usually referred to as diffuse leptomeningeal dissemination and may be less common in VHL-related HB due to its tendency toward multiplicity. Hemangioblastomatosis has been reported in several studies.[
The first two cases of disseminated sporadic HB were reported by Mohan et al., in 1976.[
The mean age of patients at the first surgery was 44.8 years old (range, 4–75);[
In total, there were 18 men and 12 women. Most patients underwent initial resection of a posterior fossa HB; two patients were initially diagnosed with cervical spine HB,[
In most cases, craniospinal dissemination was observed with the involvement of all neuroaxis, but in two cases, the dissemination was restricted to the brain,[
There was also great variety among the studies regarding the interval to the diagnosis of CNS dissemination. The interval ranged from 1 month[
In many studies, patients died within 4 years of the diagnosis of CNS dissemination.[
Regarding adjuvant treatment, there was great heterogeneity among the studies, probably due to the rarity of this condition and the lack of evidence favoring adjuvant treatment. Surgical treatment was performed in all patients, and brain and spinal radiotherapy or radiosurgery were chosen as adjuvant treatments in many studies but with variable clinical and radiological outcomes. Chemotherapy was used in only five studies. One patient was treated with Interferon-2 α and minocycline,[
Vascular endothelial growth factor (VEGF) and VEGF receptors are expressed in HBs, and antiangiogenic therapy is now being considered as a therapeutic option for some patients.[
Lesions that are not surgically accessible can be treated with stereotactic radiosurgery and radiotherapy.[
In VHL-related HBs, surgical treatment should be reserved for symptomatic lesions due to the unpredictable progression of tumors and to prevent unnecessary neurological dysfunction. Stereotactic radiosurgery can be an option for surgically inaccessible tumors and for patients who cannot tolerate surgery; however, it has shown similar tumor progression when compared to the natural history of untreated tumors after 7 years.[
CONCLUSION
Dissemination of sporadic HB is rare and aggressive disease evolution is usually observed. Further studies are necessary to determine the optimal therapeutic options.
Compliance with ethical standards
The patient’s relatives consented to the submission of the case report to the journal. The case report was written in accordance with the COPE guidelines and complies with the CARE statement. The authors declare no conflicts of interest. The authors have no relevant financial or nonfinancial interests to disclose.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Akimoto J, Fukuhara H, Suda T, Nagai K, Hashimoto R, Michihiro K. Disseminated cerebellar hemangioblastoma in two patients without von Hippel-Lindau disease. Surg Neurol Int. 2014. 5: 145
2. Amelot A, Bouazza S, Polivka M, George B, Bresson D. Sporadically second localization of cerebellar hemangioblastoma in sella turcica mimicking a meningioma with no associated von Hippel-Lindau disease. Br J Neurosurg. 2015. 29: 589-91
3. Badran A, Shepard MJ, Ksendzovsky A, Murayi R, Hayes C, Smart D. Hemangioblastomatosis-associated negative-pressure hydrocephalus managed with improvised shunt. J Clin Neurosci. 2018. 58: 226-8
4. Bains SJ, Niehusmann PF, Meling TR, Saxhaug C, Züchner M, Brandal P. Disseminated central nervous system hemangioblastoma in a patient with no clinical or genetic evidence of von Hippel-Lindau disease-a case report and literature review. Acta Neurochir (Wien). 2019. 161: 343-9
5. Bamps S, Calenbergh FV, Vleeschouwer SD, Loon JV, Sciot R, Legius E. What the neurosurgeon should know about hemangioblastoma, both sporadic and in Von Hippel-Lindau disease: A literature review. Surg Neurol Int. 2013. 4: 145
6. Chung SY, Jeun SS, Park JH. Disseminated hemangioblastoma of the central nervous system without Von Hippel-Lindau disease. Brain Tumor Res Treat. 2014. 2: 96-101
7. Courcoutsakis NA, Prassopoulos PK, Patronas NJ. Aggressive leptomeningeal hemangioblastomatosis of the central nervous system in a patient with von Hippel-Lindau disease. AJNR Am J Neuroradiol. 2009. 30: 758-60
8. Cuccarini V, Tringali G, Anghileri E. Central nervous system non-von Hippel-Lindau (VHL) hemangioblastoma evolving to dissemination: Is anti-angiogenic therapy an option?. Arch Clin Med Case Rep. 2021. 5: 303-13
9. Dornbos D, Kim HJ, Butman JA, Lonser RR. Review of the neurological implications of von Hippel-Lindau disease. JAMA Neurol. 2018. 75: 620-7
10. Franco A, Pytel P, Lukas RV, Chennamaneni R, Collins JM. CNS hemangioblastomatosis in a patient without von HippelLindau disease. CNS Oncol. 2017. 6: 101-5
11. Gläsker S, Vergauwen E, Koch CA, Kutikov A, Vortmeyer AO. Von Hippel-Lindau disease: Current challenges and future prospects. Onco Targets Ther. 2020. 13: 5669-90
12. Halim M, Halim A, Trivosa V. Symptoms, diagnosis and treatment of hemangioblastoma dissemination. OAJ Radiol. 2019. 3: 1-10
13. Hande AM, Nagpal RD. Cerebellar haemangioblastoma with extensive dissemination. Br J Neurosurg. 1996. 10: 507-11
14. Hanse MC, Vincent A, van den Bent MJ. Hemangioblastomatosis in a patient with von Hippel-Lindau disease. J Neurooncol. 2007. 82: 163-4
15. Kato M, Ohe N, Okumura A, Shinoda J, Nomura A, Shuin T. Hemangioblastomatosis of the central nervous system without von Hippel-Lindau disease: A case report. J Neurooncol. 2005. 72: 267-70
16. Kim BY, Jonasch E, McCutcheon IE. Pazopanib therapy for cerebellar hemangioblastomas in von Hippel-Lindau disease: Case report. Target Oncol. 2012. 7: 145-9
17. Kim HR, Suh YL, Kim JW, Lee JI. Disseminated hemangioblastomatosis of the central nervous system without von Hippel-Lindau disease: A case report. J Korean Med Sci. 2009. 24: 755-9
18. Knoop N, Seidel C, Frydrychowicz C, Meixensberger J. Combined microsurgery and radiotherapy for multiple spinal cord hemangioblastomas with holocord syrinx in von HippelLindau disease: A case report. J Neurol Surg Rep. 2019. 80: e46-50
19. Koo HW, Park JE, Cha J, Kim DJ, Kang SG, Lim SC. Hemangioblastomas with leptomeningeal dissemination: Case series and review of the literature. Acta Neurochir (Wien). 2016. 158: 1169-78
20. Lightfoot NJ, Lucas PG, Finnis ND. Disseminated haemangioblastoma without evidence of the von HippelLindau syndrome or haemangioblastomatosis--A case report and clinico-pathological correlation. Clin Neurol Neurosurg. 2007. 109: 305-10
21. Migliorini D, Haller S, Merkler D, Pugliesi-Rinaldi A, Koka A, Schaller K. Recurrent multiple CNS hemangioblastomas with VHL disease treated with pazopanib: A case report and literature review. CNS Oncol. 2015. 4: 387-92
22. Mohan J, Brownell B, Oppenheimer DR. Malignant spread of haemangioblastoma: Report on two cases. J Neurol Neurosurg Psychiatry. 1976. 39: 515-25
23. Ohba H, Yamaguchi S, Magaki T, Takeda M, Kolakshyapati M, Sadatomo T. A Case of holocord leptomeningeal dissemination from cerebellar hemangioblastoma without von Hippel-Lindau disease. Hiroshima J Med Sci. 2017. 66: 7-10
24. Park JG, Song SW, Koh YC. A very aggressive hemangioblastomatosis without VHL gene mutation. Nerve. 2015. 1: 44-6
25. 25. Raghavan R, Krumerman J, Rushing EJ, White CL, Chason DP, Watson ML. Recurrent (nonfamilial) hemangioblastomas involving spinal nerve roots: Case report. Neurosurgery. 2000. 47: 1443-8
26. Ramachandran R, Lee HS, Matthews B, Shatzel A, Tihan T. Intradural extramedullary leptomeningeal hemangioblastomatosis and paraneoplastic limbic encephalitis diagnosed at autopsy: An unlikely pair. Arch Pathol Lab Med. 2008. 132: 104-8
27. Reyes-Botero G, Pérez-Larraya JG, Sanson M. Sporadic CNS hemangioblastomatosis, response to sunitinib and secondary polycythemia. J Neurooncol. 2012. 107: 439-40
28. Reyns N, Assaker R, Louis E, Lejeune JP. Leptomeningeal hemangioblastomatosis in a case of von Hippel-Lindau disease: Case report. Neurosurgery. 2003. 52: 1212-5
29. Salim DK. Continuous angiogenesis inhibition in the treatment for von Hippel-Lindau-related hemangioblastomas of retina and spinal cord. J Oncol Pharm Pract. 2019. 25: 2049-51
30. Seystahl K, Weller M, Bozinov O, Reimann R, Rushing E. Neuropathological characteristics of progression after prolonged response to bevacizumab in multifocal hemangioblastoma. Oncol Res Treat. 2014. 37: 209-12
31. Taylor DG, Ilyas A, Mehta GU, Chen CJ, Schiff D, Oldfield EH. Variable response of CNS hemangioblastomas to Pazopanib in a single patient with von Hippel-Lindau disease: Case report. J Clin Neurosci. 2018. 50: 154-6
32. Tohyama T, Kubo O, Kusano R, Miura N, Himuro H. A case of hemangioblastoma with subarachnoid dissemination. No Shinkei Geka. 1990. 18: 83-8
33. Weil RJ, Vortmeyer AO, Zhuang Z, Pack SD, Theodore N, Erickson RK. Clinical and molecular analysis of disseminated hemangioblastomatosis of the central nervous system in patients without von Hippel-Lindau disease. Report of four cases. J Neurosurg. 2002. 96: 775-87
34. Zhang Q, Ma L, Li WY, Chen J, Ju Y, Hui XH. Von HippelLindau disease manifesting disseminated leptomeningeal hemangioblastomatosis: Surgery or medication?. Acta Neurochir (Wien). 2011. 153: 48-52