- Department of Neurosurgery Hospital Privado de Rosario, Rosario, Santa Fe, Argentina
- Department of Neuroradiology, Hospital Privado de Rosario, Rosario, Santa Fe, Argentina
- Department of Surgical Neuroanatomy Laboratory, Department of Anatomy, University of Buenos Aires, Argentina,
- Department of Neuropsychology, Hospital Privado de Rosario, Rosario, Santa Fe, Argentina.
Correspondence Address:
Ignacio J. Barrenechea
Department of Neuropsychology, Hospital Privado de Rosario, Rosario, Santa Fe, Argentina.
DOI:10.25259/SNI_608_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ignacio J. Barrenechea1, Luis Márquez1, Sabrina Miralles2, Matias Baldoncini3, Silvina Peralta4. An alternative path to atrial lesions through a contralateral interhemispheric transfalcine transcingular infra-precuneus approach: A case report. 25-Nov-2020;11:407
How to cite this URL: Ignacio J. Barrenechea1, Luis Márquez1, Sabrina Miralles2, Matias Baldoncini3, Silvina Peralta4. An alternative path to atrial lesions through a contralateral interhemispheric transfalcine transcingular infra-precuneus approach: A case report. 25-Nov-2020;11:407. Available from: https://surgicalneurologyint.com/surgicalint-articles/10412/
Abstract
Background: The surgical management of lesions located in the trigone of the lateral ventricle remains a neurosurgical challenge. Previously described approaches to the atrium include the transtemporal, parietal transcortical, parietal trans intraparietal sulcus, occipital transcingulate, posterior transcallosal, and transfalcine transprecuneus. However, reaching this area specifically through the cingulate cortex below the subparietal sulcus has not been described thus far.
Case Description: We present here the removal of a left atrial meningioma through a right parietal “contralateral interhemispheric transfalcine transcingular infra-precuneus” approach and compare it with previously described midline approaches to the atrium. To accomplish this, a right parietal craniotomy was performed. After the left subprecuneus cingulate cortex was exposed through a window in the falx, a limited corticotomy was performed, which allowed the tumor to be reached after deepening the bipolar dissection by 8 mm. Postoperative magnetic resonance imaging showed complete resection of the lesion sparing the corpus callosum, forceps major, and sagittal stratum. Although this approach disrupts the posterior cingulate fasciculus, no deficits have been described so far after unilaterally disrupting the posterior cingulate cortex or the posterior part of the cingulate fasciculus. In fact, a thorough postoperative cognitive examination did not show any deficits.
Conclusion: The “contralateral interhemispheric transfalcine transcingular infra-precuneus” approach combines the advantages of several previously described approaches. Since it conserves the major white matter tracts that surround the atrium and has a shorter attack angle than the contralateral transfalcine transprecuneus approach, we believe that it could be a potentially new alternative path to reach atrial lesions.
Keywords: Atrium of the lateral ventricle, Case report, Contralateral interhemispheric approach, Posterior cingulate cortex, Tumor
INTRODUCTION
The surgical management of lesions located in the ventricular atrium remains a neurosurgical challenge. The deep location, eloquence of surrounding cortices, and close relationship with important white matter tracts make the approach to this area challenging. Considering that these white matter fibers are mainly on the lateral surface of the atrium, many neurosurgeons have shifted to midline approaches to reach this area. Thus, approaches through the precuneus or the isthmus of the cingulum are gaining acceptance within the neurosurgical community.[
CASE ILLUSTRATION
We present the case of a 56-year-old female who had been treated for uterine cervical cancer for the past 2 years. During a follow-up positron emission tomography-computed tomography (CT) scan, a hypermetabolic lesion was found in her left brain hemisphere. Further studies showed a lesion in her left atrium, which was homogeneously enhanced after gadolinium administration [
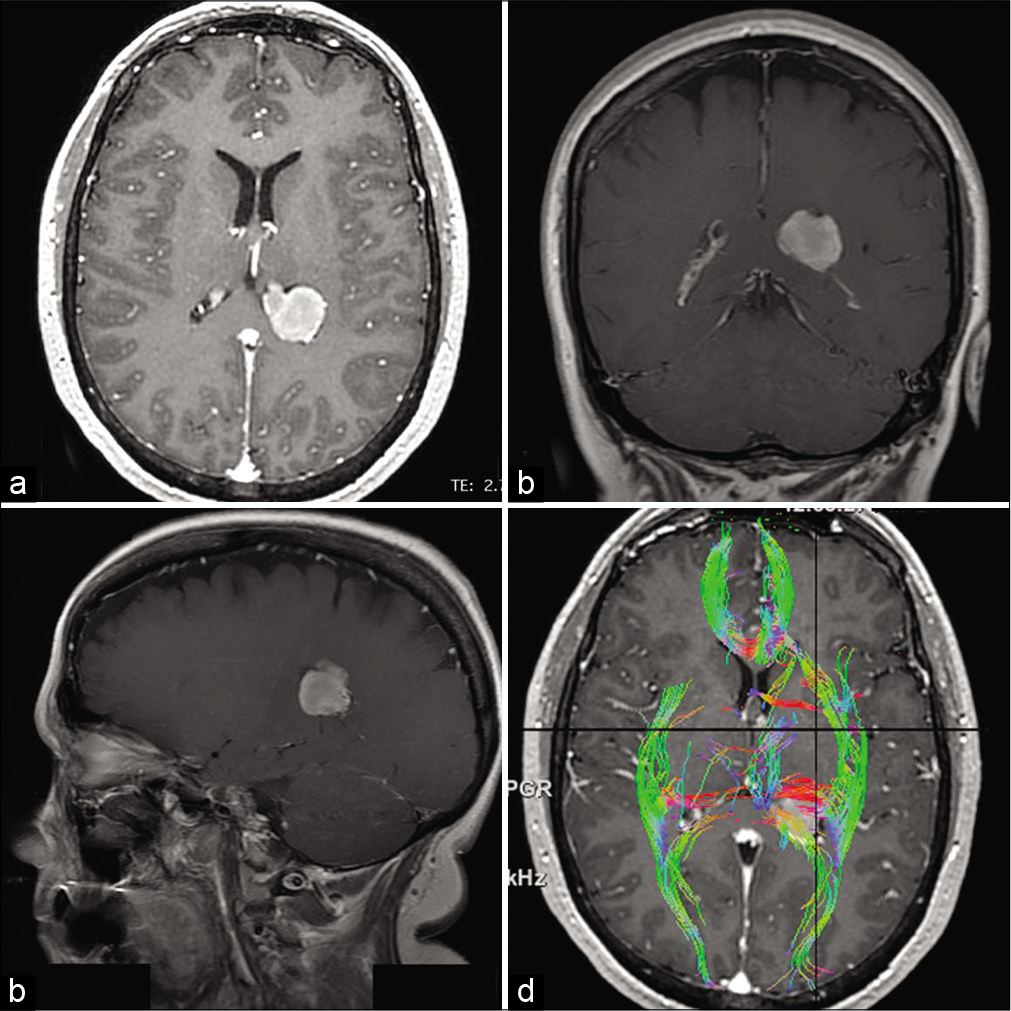
Figure 1:
(a) Axial T1-weighted contrast-enhanced magnetic resonance imaging (MRI) revealing a 4 cm homogeneously enhancing mass in the atrium of the left lateral ventricle. (b and c) Coronal and sagittal T1-weighted contrast-enhanced MRI showing the tumor. (d) Diffusion tensor imaging superimposed on an axial T1-weighted contrast-enhanced MRI, depicting the tumor surrounded laterally by the sagittal stratum, anterolaterally by the internal capsule, and posteromedially by the splenium of the corpus callosum and forceps major.
Technique
After general anesthesia was induced, arterial and central lines were introduced. The patient was placed in a right three-quarter prone position, with her head rotated 90° clockwise from vertical (to orient the midline horizontally) and secured in a Mayfield head holder. Optical neuronavigation (Tracker Navigation System, Física Médica SRL, Córdoba, Argentina) was introduced to aid in the design of the skin incision and plan the trajectory to the contralateral atrium. A right parietal horseshoe incision and parietal craniotomy were performed, to allow gravity to retract the right parietal lobe. In this way, the contralateral atrial tumor was in line with the surgeon’s line of view [
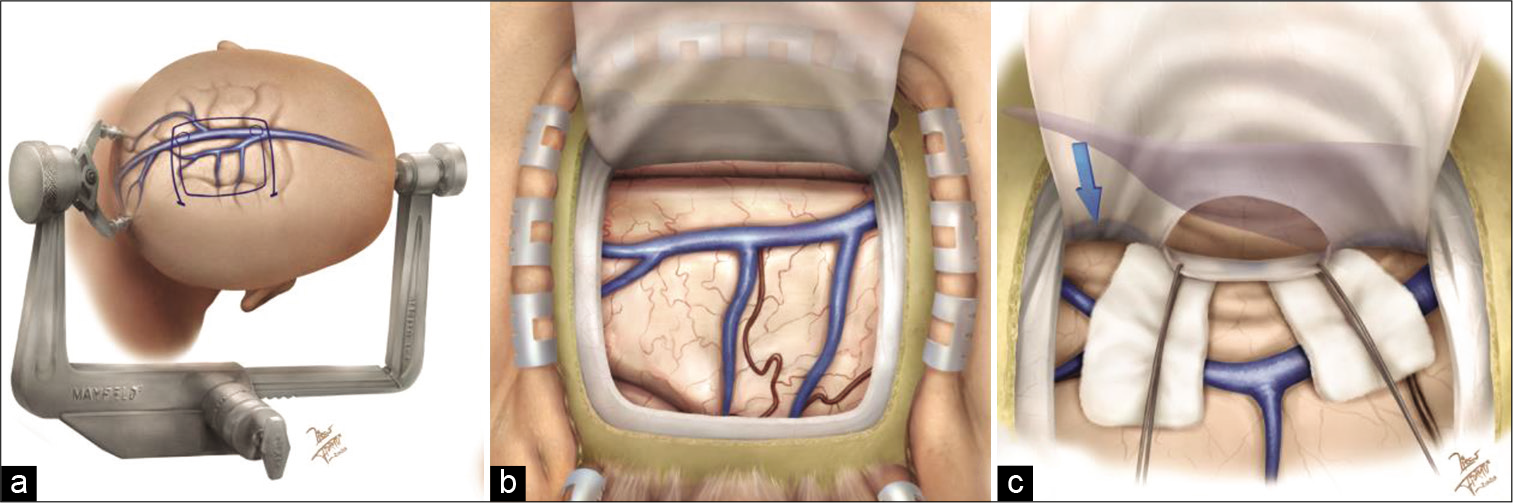
Figure 2:
(a) Artistic rendering showing the patient’s head position, skin incision, and craniotomy outline. This position allows the use of gravity retraction to mobilize the right hemisphere away from the falx, thereby avoiding the need for self-retaining retractors on the left hemisphere. (b) Initial exposure of the right parietal lobe. The vein anatomy of this area should be thoroughly studied preoperatively. (c) A window in the falx above the inferior sagittal sinus (blue arrow) has been created. This window exposes the cingulate cortex below the subparietal sulcus. The lateral ventricle and atrium are depicted in transparency.
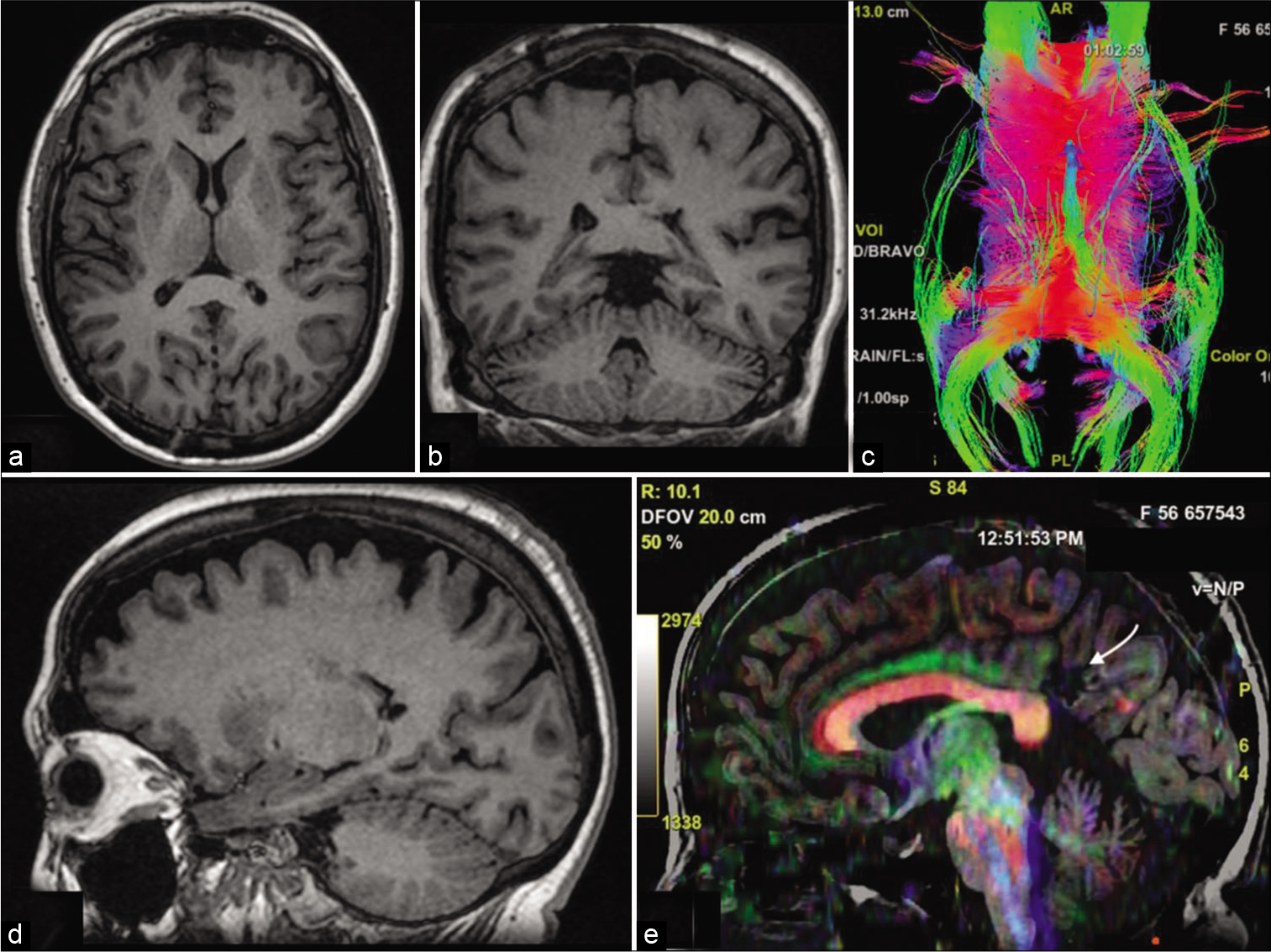
Figure 4:
(a and b) Six-month postoperative axial and coronal T1-weighted magnetic resonance imaging (MRI) showing complete resection of the tumor. (c) Postoperative tractography depicting the sparing of the fornix, forceps major, and splenium of the corpus callosum, with a small window in the left tapetum. (d) Postoperative sagittal T1-weighted MRI. (e) Sagittal diffusion tensor imaging depicting the corticotomy area and the attack angle on the left dorsal posterior cingulate cortex (white arrow).
DISCUSSION
Previously described approaches to the trigone include the transtemporal, parietal transcortical, parietal trans intraparietal sulcus, occipital transcingulate, and posterior transcallosal and the recently described transfalcine transprecuneus approach.[
The intraparietal transsulcal approach has been traditionally preferred for its direct access to lesions in the trigone of the lateral ventricle. However, this approach has been associated with neurological deficits, including apraxia, acalculia, and visual field deficits, of which the most common is homonymous hemianopsia.[
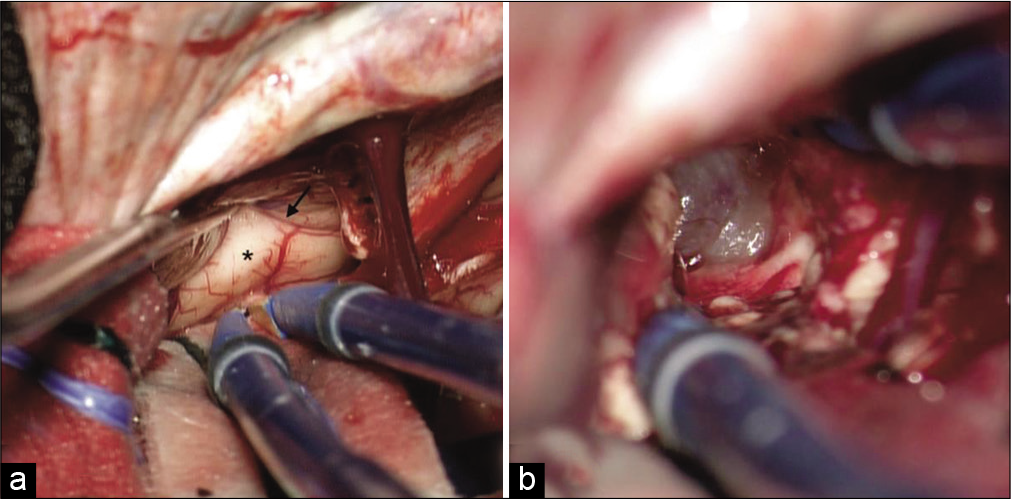
Figure 5:
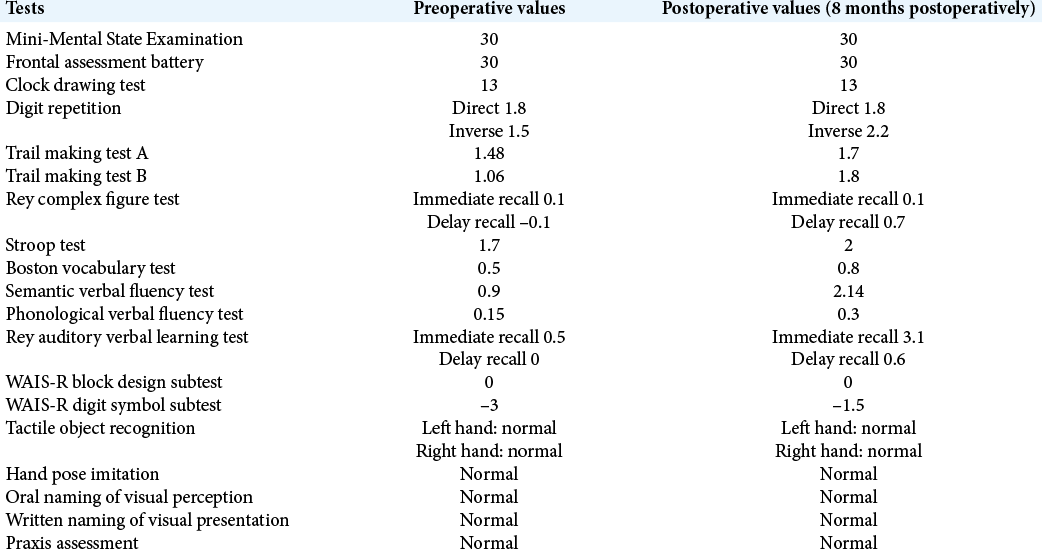
(a) Cadaveric laboratory dissection of the medial aspect of the right hemisphere through a window in the falx. P: Precuneus, S: Splenium of the corpus callosum, dPCC: Dorsal posterior cingulate cortex. Curved black arrow: subparietal sulcus. (b) Stepwise dissection through the dPCC reveals the cingulate bundle (CB). (c) Exposure of the atrium of the right lateral ventricle (A). An arterial branch is seen in the subparietal sulcus (straight black arrow). This vessel could be potentially injured in a transprecuneus approach. (d) Visualization of the glomus of the choroid plexus (G). The inferior sagittal sinus has been cut only for teaching purposes. This maneuver was not necessary during the procedure.
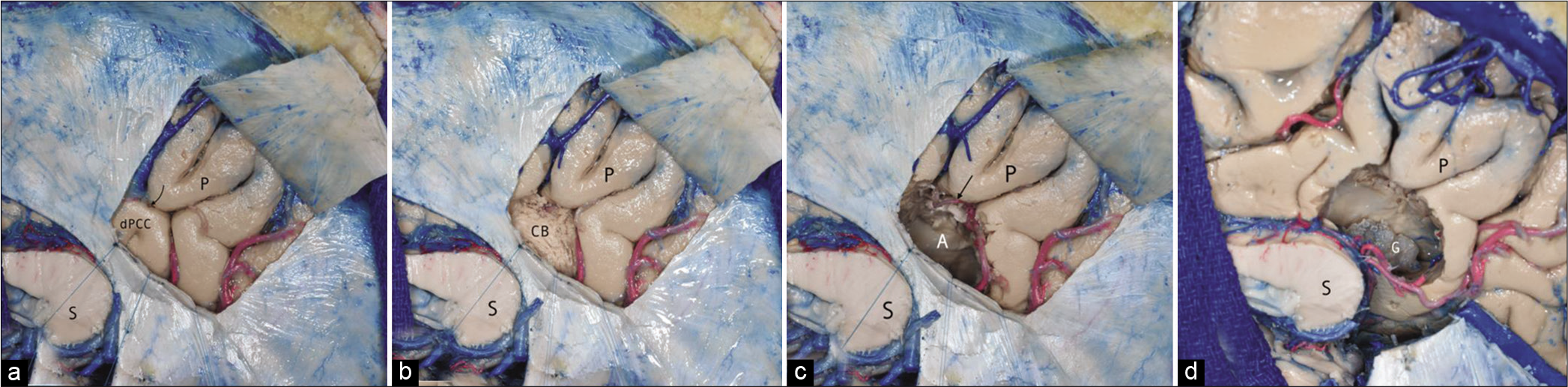
Figure 6:
(a) Artistic rendering showing the approximate locations of posterior cingulate cortex subdivisions based on cytoarchitectonics, with associated Brodmann areas. (b) Artistic rendering comparing the corticotomy location of the CITTI approach with the previously described posterior interhemispheric transfalcine transprecuneus approach (PITTA) and the ipsilateral occipital transcingulate approach. (c and d) Sagittal T1-weighted images comparing the trajectories of the CITTI versus PITTA approach. (c) Measures were taken from the cortices of the precuneus and the dorsal posterior cingulate cortex to the tumor. (d) Note the shorter intraparenchymal path from the cortex to the atrial tumor (highlighted in green) through the CITTI approach (8 mm) compared with the PITTA approach (19 mm). In addition, the subparietal sulcus (with its vessels) is preserved with our approach.
Thus, this approach has a real advantage over the intraparietal transsulcal approach. However, we must acknowledge that we have removed a medium-sized tumor through this approach. Tumors larger than 5 cm projecting higher than the subparietal sulcus might require cutting into the precuneus for removal. Furthermore, the presence of numerous large parasagittal veins draining into the sagittal sinus overlying the contralateral hemisphere should preclude the use of either this or the PITTA approach, because they could potentially jeopardize both hemispheres. Finally, utilizing neuronavigation is necessary, since it is difficult to get a proper orientation before opening the falx in a location where the inferior sagittal sinus joins the straight sinus.
CONCLUSION
The “contralateral interhemispheric transfalcine transcingular infra-precuneus” approach combines the advantages of several previously described approaches. Since it conserves the major white matter tracts that surround the atrium and has a shorter attack angle than the “PITTA” approach, we believe that it could be a potential new path to reach atrial lesions.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Belykh E, Yagmurlu K, Lei T, Safavi-Abbasi S, Oppenlander ME, Martirosyan NL. Quantitative anatomical comparison of the ipsilateral and contralateral interhemispheric transcallosal approaches to the lateral ventricle. J Neurosurg. 2018. 128: 1492-502
2. Bohnstedt BN, Kulwin CG, Shah MV, Cohen-Gadol AA. Posterior interhemispheric transfalcine transprecuneus approach for microsurgical resection of periatrial lesions: Indications, technique, and outcomes. J Neurosurg. 2015. 123: 1045-54
3. Bubb EJ, Metzler-Baddeley C, Aggleton JP. The cingulum bundle: Anatomy, function, and dysfunction. Neurosci Biobehav Rev. 2018. 92: 104-27
4. Caruana F, Gerbella M, Avanzini P, Gozzo F, Pelliccia V, Mai R. Motor and emotional behaviours elicited by electrical stimulation of the human cingulate cortex. Brain. 2018. 141: 3035-51
5. Davies J, Tawk RG, Lawton MT. The contralateral transcingulate approach. Oper Neurosurg. 2012. 71: ONS4-14
6. Kawashima M, Li X, Rhoton AL, Ulm AJ, Oka H, Fujii K. Surgical approaches to the atrium of the lateral ventricle: Microsurgical anatomy. Surg Neurol. 2006. 65: 436-45
7. Koutsarnakis C, Liakos F, Kalyvas AV, Liouta E, Emelifeonwu J, Kalamatianos T. Approaching the atrium through the intraparietal sulcus: Mapping the sulcal morphology and correlating the surgical corridor to underlying fiber tracts. Oper Neurosurg (Hagerstown). 2017. 13: 503-16
8. Lawton MT, Golfinos JG, Spetzler RF. The contralateral transcallosal approach: Experience with 32 patients. Neurosurgery. 1996. 39: 729-34
9. Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014. 137: 12-32
10. Mahaney KB, Abdulrauf SI. Anatomic relationship of the optic radiations to the atrium of the lateral ventricle: Description of a novel entry point to the trigone. Neurosurgery. 2008. 63: 195-202
11. Rowe R. Surgical approaches to the trigone. Contemp Neurosurg. 2005. 27: 1-5
12. Sun C, Xie T, Zhang X, Zhu W, Gu Y, Wang H. To repeat or to recreate: A contralateral posterior interhemispheric transfalcine transprecuneus approach for recurrent meningiomas at the trigone of the lateral ventricle. J Clin Neurosci. 2014. 21: 1968-72
13. Vogt BA, Vogt BA.editors. Regions and subregions of the cingulate cortex. Cingulate Neurobiology and Disease. New York: Oxford University Press Inc; 2009. p. 3-30
14. Vogt BA.editors. Structural Organization of Cingulate Cortex: Areas, Neurons, and Somatodendritic Transmitter Receptors, in Neurobiology of Cingulate Cortex and Limbic Thalamus. Boston, MA: Birkhäuser Boston; 1993. p. 19-70
15. Wang S, Salma A, Ammirati M. Posterior interhemispheric transfalx transprecuneus approach to the atrium of the lateral ventricle: A cadaveric study. J Neurosurg. 2010. 113: 949-54
16. Yaşargil MG, Abdulrauf SI. Surgery of intraventricular tumors. Neurosurgery. 2008. 62: 1029-41
17. Yaşargil MG, Türe U, Yaşargil DC. Surgical anatomy of supratentorial midline lesions. Neurosurg Focus. 2005. 18: E1
18. Yasargil MG. Intraventricular Tumors, in Georg Thieme Verlag: CNS Tumors: Surgical Anatomy, Neuropathology, Neuroradiology, Neurophysiology, Clinical Considerations, Operability, Treatment Options. Stuttgart: Thieme Publisher Series; 1994. 4: 313-38
19. Zaidi HA, Chowdhry SA, Nakaji P, Abla AA, Spetzler RF. Contralateral interhemispheric approach to deep-seated cavernous malformations: Surgical considerations and clinical outcomes in 31 consecutive cases. Neurosurgery. 2014. 75: 80-6
20. Zhu W, Xie T, Zhang X, Ma B, Wang X, Gu Y. A solution to meningiomas at the trigone of the lateral ventricle using a contralateral transfalcine approach. World Neurosurg. 2013. 80: 167-72






James Ausman
Posted November 30, 2020, 8:39 am
Excellent approach for this problem. You may be interested in this paper: Three-Quarter Prone Approach to the Pineal-Tentorial Region Ausman et al Surg Neurol 1988;29:298-306
Ignacio Barrenechea, MD, IFAANS
Posted January 4, 2021, 7:52 am
Dear Dr. Ausman: Thank you very much for your comments! They mean a lot to me! I will certainly look into the suggested paper! Best Regards,