- Department of Neurosurgery, Shinshu University, Matsumoto, Nagano, Japan.
- Departments of Neurosurgery, Shinshu University School of Medicine, Matsumoto, Nagano, Japan.
- Departments of Laboratory Medicine, Shinshu University School of Medicine, Matsumoto, Nagano, Japan.
- Pathology, Shinshu University School of Medicine, Matsumoto, Nagano, Japan.
Correspondence Address:
Tetsuya Goto
Departments of Neurosurgery, Shinshu University School of Medicine, Matsumoto, Nagano, Japan.
DOI:10.25259/SNI-183-2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ridzky Firmansyah Hardian, Tetsuya Goto, Haruki Kuwabara, Yoshiki Hanaoka, Shota Kobayashi, Hiroyuki Kanno, Hisashi Shimojo, Tetsuyoshi Horiuchi, Kazuhiro Hongo. An autopsy case of widespread brain dissemination of glioblastoma unnoticed by magnetic resonance imaging after treatment with bevacizumab. 05-Jul-2019;10:137
How to cite this URL: Ridzky Firmansyah Hardian, Tetsuya Goto, Haruki Kuwabara, Yoshiki Hanaoka, Shota Kobayashi, Hiroyuki Kanno, Hisashi Shimojo, Tetsuyoshi Horiuchi, Kazuhiro Hongo. An autopsy case of widespread brain dissemination of glioblastoma unnoticed by magnetic resonance imaging after treatment with bevacizumab. 05-Jul-2019;10:137. Available from: http://surgicalneurologyint.com/surgicalint-articles/9458/
Abstract
Background: Although glioblastoma has been shown to be able to disseminate widely in the intracranially after treatment with bevacizumab without any significant radiological findings, reports on such cases with subsequent autopsy findings are lacking.
Case Description: A 36-year-old man presented with a general seizure and a mass of the right frontal lobe, which was diagnosed as diffuse astrocytoma (WHO Grade II). The patient underwent a total of four surgeries from 2005 to 2017. He showed tumor recurrence, progression, and malignant transformation to glioblastoma (GBM) (WHO Grade IV) despite repeated tumor resections, radiotherapy, and chemotherapies with temozolomide and carmustine wafers. Bevacizumab (10 mg/kg body weight) was started following the fourth surgery. After bevacizumab administration, the patient’s clinical condition improved to a Karnofsky performance status (KPS) score of 50–60, and he was stable for several months before finally deteriorating and passing away. Although sequential magnetic resonance imaging (MRI) showed shrinkage of the lesion and a reduction of edema, an autopsy showed widespread tumor invasion that was not revealed on MRI. Neoplastic foci were identified extensively in the cerebral cortex, basal ganglia, pituitary gland, cerebellum, and brainstem, imposing as gliomatosis cerebri.
Conclusion: Imaging follow-up of malignant gliomas needs to be interpreted with caution as marked improvement in radiological response after bevacizumab treatment may not be indicating tumor regression. Despite the notable lack of evidence to increase overall survival in GBM patients with bevacizumab, the increase in progression-free survival and the observed relief of symptoms due to a decrease in edema should be considered relevant for patient management.
Keywords: Antiangiogenesis agent, Autopsy, Bevacizumab, Brain tumor, Chemotherapy, Glioblastoma
INTRODUCTION
Glioblastoma (GBM) is the most common primary malignancy of the central nervous system in adults.[
High angiogenicity in GBM is contributed by an overexpression of vascular endothelial growth factor (VEGF).[
In this paper, we report a case of a patient with initial low- grade glioma who underwent a series of surgeries from 2005 to 2017 before he developed secondary GBM treated with bevacizumab. Subsequent autopsy study showed tumor invasion adjacent to the resection cavity that was not revealed in postoperative magnetic resonance imaging (MRI). The patient could maintain a relatively good clinical condition in spite of worsening disease progression. Although previous studies suggested the possibility of tumor masking in cases GBM treated with bevacizumab,[
CASE REPORT
A 36-year-old man presented to our hospital in April 2005 with a general seizure. Fluid-attenuated inversion recovery (FLAIR) MRI of the brain showed a hyperintense lesion in the right frontal lobe suspicious for a low-grade glioma [
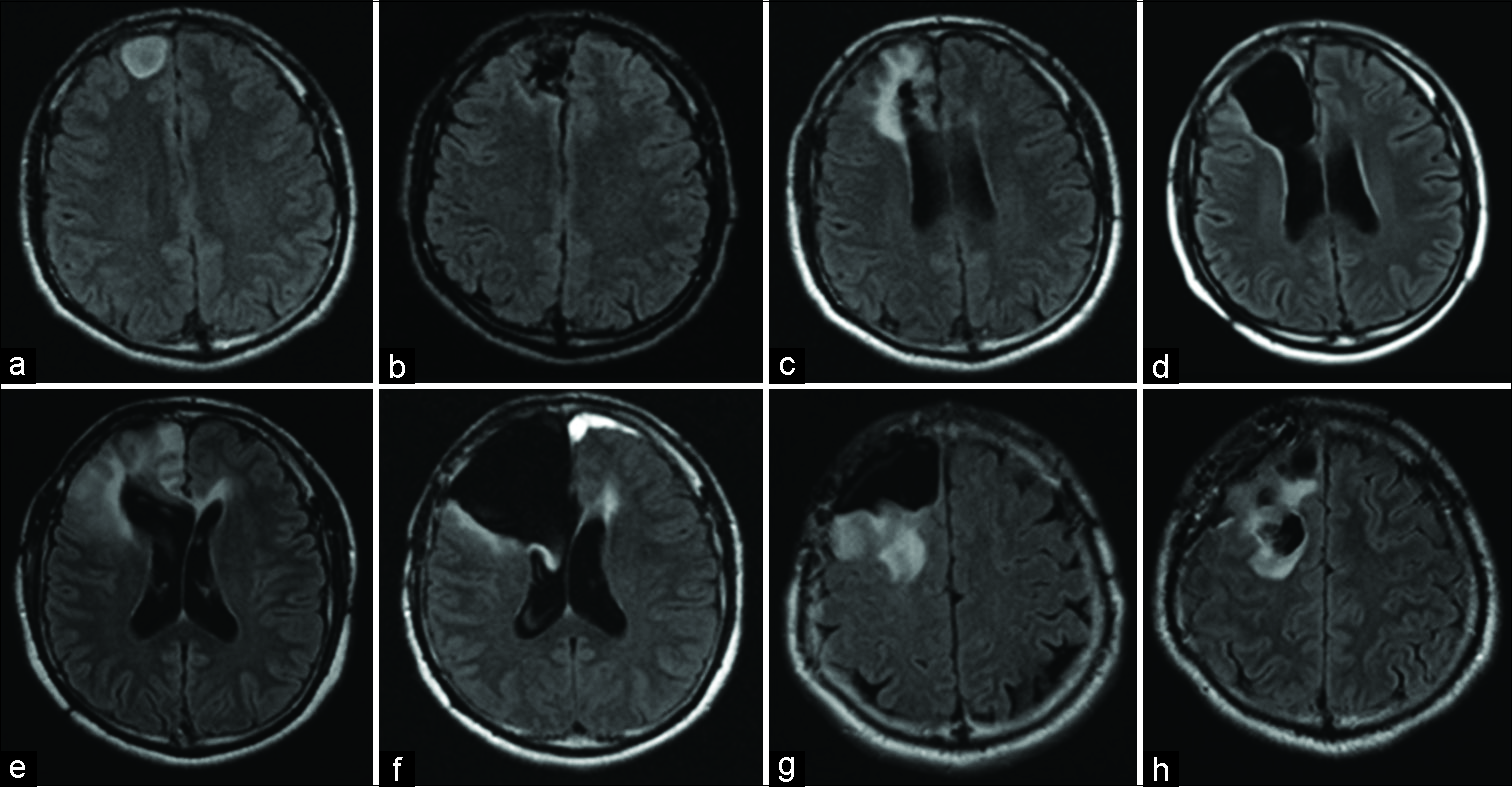
Figure 1:
Axial fluid-attenuated inversion recovery (FLAIR) magnetic resonance imaging (MRI) demonstrating progression of the lesion over the patient clinical course. (a) The initial MRI before the first operation showing a hyperintense lesion in the right frontal lobe. (b) Postoperative MRI of the first surgery showing total removal of the FLAIR high lesion. (c) MRI of 5 years after the first operation demonstrating recurrence of the lesion around the removal cavity. (d) Postoperative MRI showing total removal of the recurring lesion. (e) MRI at 4 years after the second surgery showing another recurrent lesion in the right lower frontal lobe. (f) Postoperative MRI of the third surgery showing residual hyperintense lesion in the medial side of the temporal lobe. (g) MRI 2 years after the third operation showing hyperintense lesion reaching near the pyramidal tracts. (h) Carmustine wafers were placed in the removal cavity.
General seizures reoccurred in 2010, 5 years after the first surgery. The seizures were not controlled with antiepileptic drugs. Radiographic reassessment at this point showed a lesion adjacent to the surgical cavity indicating recurrent tumor [
Four years after the second surgery, seizure attacks again recurred frequently. A recurrent lesion appeared enlarging the bottom of the right frontal lobe [
Two years later, follow-up FLAIR MRI showed progression of the lesion extending toward the pyramidal tract [
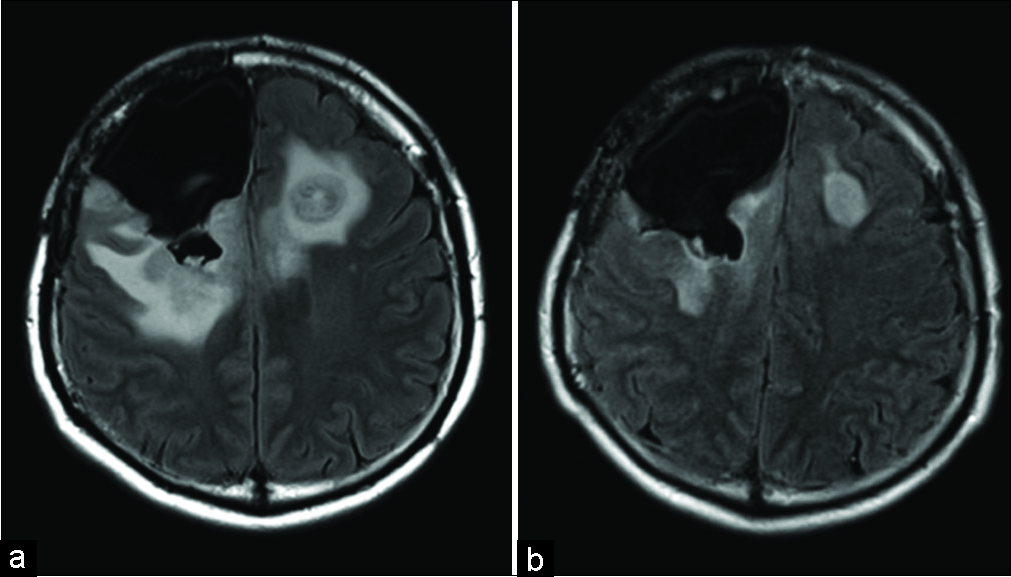
Approximately 8 weeks after the fourth operation, the right-sided paresis of the patient worsened and his general condition deteriorated rapidly. The Karnofsky performance status (KPS) score decreased from 70 to 50 and MRI disclosed that the lesion had progressed contralaterally. Due to the observed tumor progression, bevacizumab (10 mg/kg body weight) was given and within 1 month, the KPS score had improved to 60. The right-side hemiparesis improved. Radiological examination showed shrinkage of the lesion and decreased edema [
Over the course of the ensuing 5 months, bevacizumab was administered 8 times before the patient deteriorated again to terminal state with epileptic seizures occurring more frequently.
One month after hospitalization for failure to thrive, he became somnolent with unstable respirations and he developed signs of herniation. The patient was not intubated according to his living will. He died 1 h later, after a clinical course that spanned a total of 12 years.
Autopsy finding
Gross macroscopic examination
The brain weighed 1770 g, which was slightly heavier than normal. Brain edema was attributed to this finding. There was no apparent herniation identified. A surgical cavity was seen in the right frontal lobe. Black and brown masses with indistinct margins were identified in the cerebrum, ventricle, cerebellum, and brainstem.
Histopathological examination
Histopathological examination of various tumor sites revealed cells with atypically shaped nuclei [
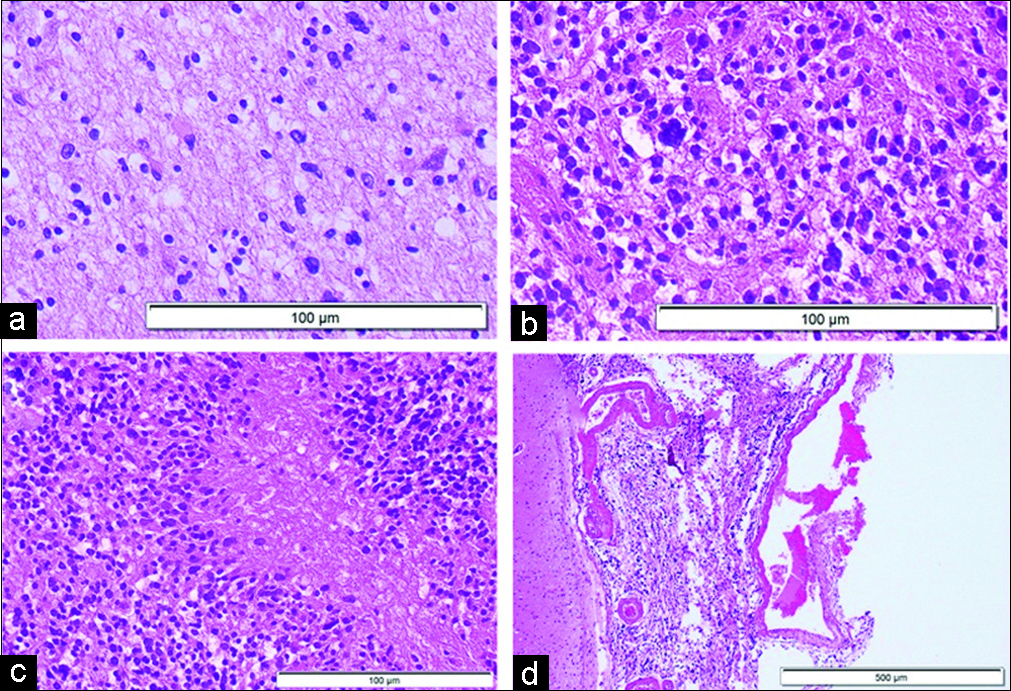
Figure 3:
Microscopic finding of autopsy in hematoxylin and eosin staining. Histopathological patterns consistent with the World Health Organization (a) Grade II, (b) Grade III, and (c) Grade IV are confirmed extensively from each part of the brain. (d) Invasion also seen in the subarachnoid space at the specimen where the arachnoid membrane was removed together with the brain tissue.
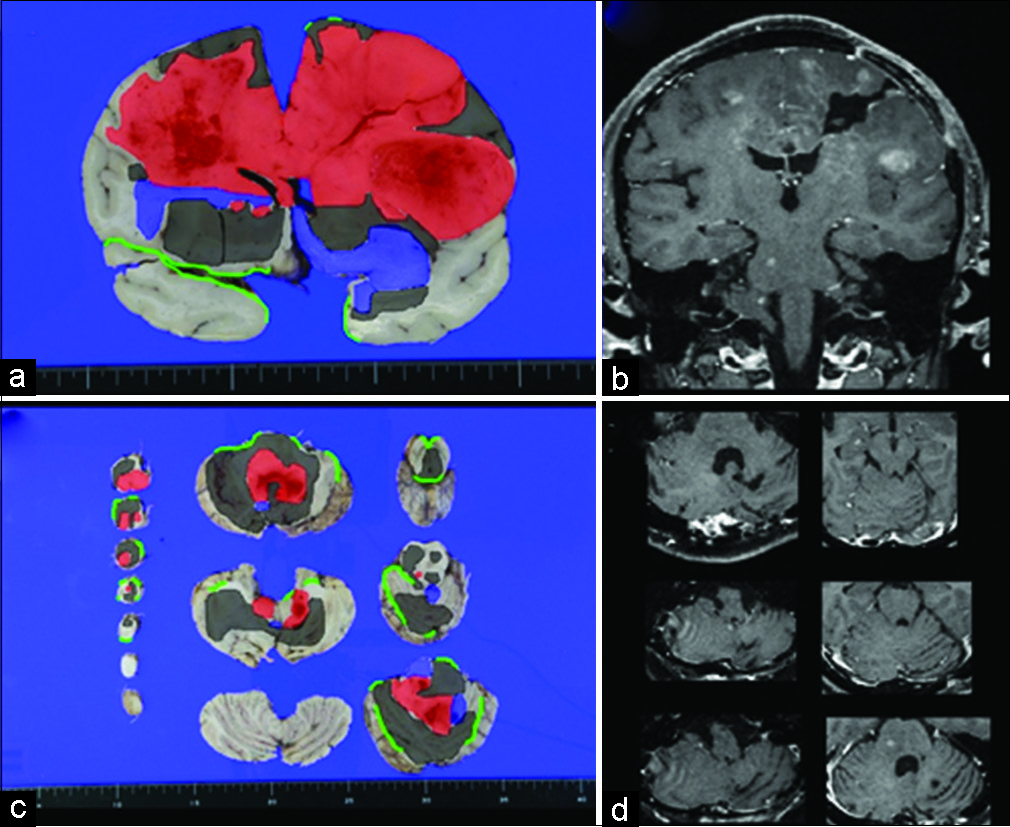
Figure 4:
Superimposition of the histopathological finding on the macroscopic photography of (a) coronal slice of the cerebrum, (c) axial slices of cerebellum and brainstem from the autopsy with corresponding T1 magnetic resonance imaging (MRI) (b and d, respectively) that taken 3 months after administration of bevacizumab. Within the superimposed images, red color indicating histopathological finding consistent with the World Health Organization (WHO) Grade IV. Blue color indicating histopathological finding consistent with the WHO Grade III. Gray color indicating histopathological finding consistent with the WHO Grade II. Green line indicating subarachnoid space. Histopathological analysis revealing widespread tumor invasion in cerebrum, cerebellum, brainstem, and pituitary region that is not seen on MRI. Around the WHO Grade IV lesion, the WHO Grade II and III lesions are widely identified, except in the superolateral part of the right temporal lobe that bordering with the right frontal lobe.
DISCUSSION
As far as our knowledge, this is one of the few reported cases demonstrating invasion of GBM to brain structures at autopsy despite brain imaging showing no signs of such tumor progression after therapy with bevacizumab. Apparent tumor size and edema decreased on brain MRI, but viable tumor cells were confirmed at autopsy when the lesion had widely spread not only within the cerebral cortex but also to the basal ganglia, cerebellum, brainstem, and pituitary gland. After administration of bevacizumab, the patient’s clinical condition slightly improved for a few months before final deterioration.
There is ongoing controversy regarding the use of bevacizumab to treat GBM. Bevacizumab is used to treat GBM due to its proposed ability to stop tumor expansion by inhibiting angiogenesis through VEGF pathway.[
GBM is characterized by high angiogenic activity with abnormal tumor blood vessels. Antiangiogenic agents seem to change tumor vessels by making them less leaky, dilated, and tortuous with more normal basement membrane and greater pericytes coverage, resulting in improved vessel perfusion.[
A number of studies suggested that angiogenesis is not the only mechanism for tumors to obtain vascular supplies because tumor cells could also recruit normal blood vessel for growth – a process known as vascular cooption.[
In this case, no tumor cells were found in the superolateral part of the right temporal lobe which was bordering the right frontal lobe where histopathological finding showed GBM. Only Grades II and III were seen in the medial part of the temporal lobe. This finding could be explained by the theory that tumor tends to spread along blood vessels or with the white matter fibers, along paths of lesser resistance.[
Normalization of tumor vascularization from bevacizumab administration appears to reduce vessel permeability and uptake of contrast-enhancing agents, thus reducing contrast enhancement usually seen in imaging modalities.[
Despite an apparent lack of evidence that bevacizumab increases OS in GBM patients, the increase in PFS and the relief of symptoms due to decreased edema should be considered relevant for patient management.[
As described in this case report, the patient could maintain a good clinical condition and stable KPS for several months despite widespread dissemination of the tumor after treatment with bevacizumab. This decrease in symptoms is important for activities of daily living and social interactions and translates in our opinion into QOL.
CONCLUSION
We reported an autopsy case of GBM in a patient who had been treated with bevacizumab in the terminal stage. The autopsy revealed that GBM had invaded widely in brain regions where radiological examination did not show any disease. Imaging follow-up needs, therefore, to be interpreted with caution as marked improvement in radiological responses after bevacizumab treatment may not reflect true antitumor effect. Despite lack of evidence for bevacizumab to increase OS in GBM patients, the increase in PFS and relief of symptoms due to decrease in edema appears relevant for the patient management.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alves TR, Lima FR, Kahn SA, Lobo D, Dubois LG, Soletti R. Glioblastoma cells: A heterogeneous and fatal tumor interacting with the parenchyma. Life Sci. 2011. 89: 532-9
2. Balana C, Penas RD, Sepúlveda JM, Gil-Gil MJ, Luque R, Gallego O. Bevacizumab and temozolomide versus temozolomide alone as neoadjuvant treatment in unresected glioblastoma: The GENOM 009 randomized phase II trial. J Neurooncol. 2016. 127: 569-79
3. Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007. 11: 83-95
4. Castro BA, Aghi MK. Bevacizumab for glioblastoma: Current indications, surgical implications, and future directions. Neurosurg Focus. 2014. 37: E9-
5. Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R. Bevacizumab plus radiotherapy temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014. 370: 709-22
6. Cloughesy T, Finocchiaro G, Belda-Iniesta C, Recht L, Brandes AA, Pineda E. Randomized, double-blind, placebo-controlled, multicenter phase II study of onartuzumab plus bevacizumab versus placebo plus bevacizumab in patients with recurrent glioblastoma: Efficacy, safety, and hepatocyte growth factor and O6methylguanine-DNA methyltransferase biomarker analyses. J Clin Oncol. 2017. 35: 343-51
7. Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009. 15: 232-9
8. Esmaeili M, Stensjøen AL, Berntsen EM, Solheim O, Reinertsen I. The direction of tumour growth in glioblastoma patients. Sci Rep. 2018. 8: 1199-
9. Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014. 370: 699-708
10. Hawkins-Daarud A, Rockne RC, Anderson AR, Swanson KR. Modeling tumor-associated edema in gliomas during anti-angiogenic therapy and its impact on imageable tumor. Front Oncol. 2013. 3: 66-
11. Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007. 8: 610-22
12. Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005. 307: 58-62
13. Leenders WP, Küsters B, Verrijp K, Maass C, Wesseling P, Heerschap A. Antiangiogenic therapy of cerebral melanoma metastases results in sustained tumor progression via vessel co-option. Clin Cancer Res. 2004. 10: 6222-30
14. Nam JY, de Groot JF. Treatment of glioblastoma. J Oncol Pract. 2017. 13: 629-38
15. Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009. 15: 220-31
16. Rubenstein JL, Kim J, Ozawa T, Zhang M, Westphal M, Deen DF. Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia. 2000. 2: 306-14
17. Sorensen AG, Batchelor TT, Wen PY, Zhang WT, Jain RK. Response criteria for glioma. Nat Clin Pract Oncol. 2008. 5: 634-44
18. Stadlbauer A, Roessler K, Zimmermann M, Buchfelder M, Kleindienst A, Doerfler A. Predicting glioblastoma response to bevacizumab through MRI biomarkers of the tumor microenvironment. Mol Imaging Biol. 2018. p.
19. Thakkar JP, Dolecek TA, Horbinski C, Ostrom QT, Lightner DD, Barnholtz-Sloan JS. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomarkers Prev. 2014. 23: 1985-96
20. Verhoeff JJ, van Tellingen O, Claes A, Stalpers LJ, van Linde ME, Richel DJ. Concerns about anti-angiogenic treatment in patients with glioblastoma multiforme. BMC Cancer. 2009. 9: 444-