- Department of Orthopaedics, Chettinad Hospital and Research Institute, Kanchipuram, Tamil Nadu, India.
DOI:10.25259/SNI_466_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: B. Yogesh Kumar, R. Thirumal, S. G. Chander. Aneurysmal bone cyst of thoracic spine with neurological deficit and its recurrence treated with multimodal intervention – A case report. 05-Sep-2020;11:274
How to cite this URL: B. Yogesh Kumar, R. Thirumal, S. G. Chander. Aneurysmal bone cyst of thoracic spine with neurological deficit and its recurrence treated with multimodal intervention – A case report. 05-Sep-2020;11:274. Available from: https://surgicalneurologyint.com/surgicalint-articles/10243/
Abstract
Background: Aneurysmal bone cysts (ABCs) are rare, representing about 1% of primary bone tumors, and 15% of all primary spine/sacral tumors. Notably, when they are located in poorly accessible regions such as the spine and pelvis, their management may be challenging. Treatment options include selective arterial embolization (SAE), curettage, en bloc excision with reconstruction, and radiotherapy.
Case Description: A 16-year-old male presented with 2 months of mid back pain, left-sided thoracic radiculopathy, and left lower limb weakness (MRC – 3/5). MR imaging revealed an expansile, lytic lesion involving the T9 vertebral body, and the left-sided posterior elements resulting in cord compression. He underwent SAE followed by intralesional excision, bone grafting, and a cage – instrumented fusion. ABC was diagnosed from the biopsy sample. Postoperatively, the pain was reduced, and he was neurologically intact. Five months later, he presented with a new lesion that was treated with repeated SAE and three doses of zoledronic acid. At the end of 2 years, the subsequent, MRI and CT studies documented new bone formation in the lytic areas, with healing of lesion; additionally, he clinically demonstrated sustained pain relief.
Conclusion: Here, we emphasized the importance of surgery for patients with ABC who develop focal neurological deficits. Treatment options should include SAE with bisphosphonate therapy for lesions that recur without neurological involvement.
Keywords: Aneurysmal bone cyst, Bisphosphonate therapy, Recurrence, Selective arterial embolization, Surgical excision
INTRODUCTION
Aneurysmal bone cysts (ABCs) are rare, locally aggressive lesions that occur most frequently in the first or second decades of life.[
CASE PRESENTATION
A 16-year-old male presented with 2 months of mid back pain and left-sided radiculopathy with the acute onset of the left lower limb monoparesis (MRC – 3/5). Other accompanying neurological findings included a T10 sensory level with loss of pin prick, temperature, vibration/position appreciation with hyperreflexia, and a left-sided positive Babinski response. The computed tomography (CT) scan of the thoracic spine demonstrated an expansile lytic lesion with the classical “egg shell layer” occupying the left side of T9 vertebral body destroying the lamina and pedicle with epidural extension [
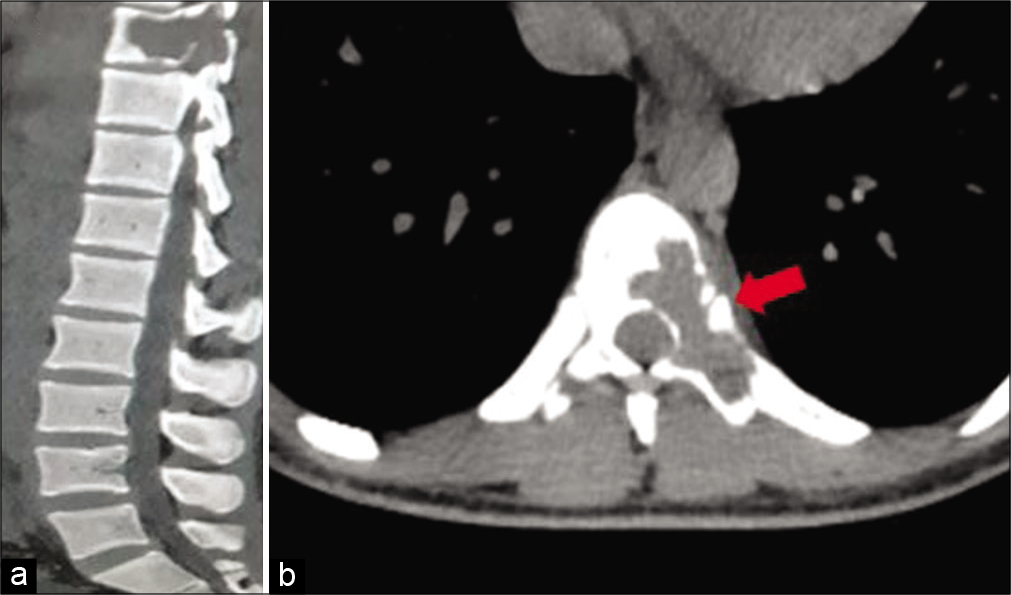
Figure 1:
Computed tomography of the spine. (a) Sagittal section showing typical expansile, osteolytic bony destruction of T9 vertebra with posterior epidural extension and cord compression. (b) Axial section showing lytic lesion with egg shell layer (marked with arrow) of the T9 body involving the left pedicle, lamina, and spinous process.
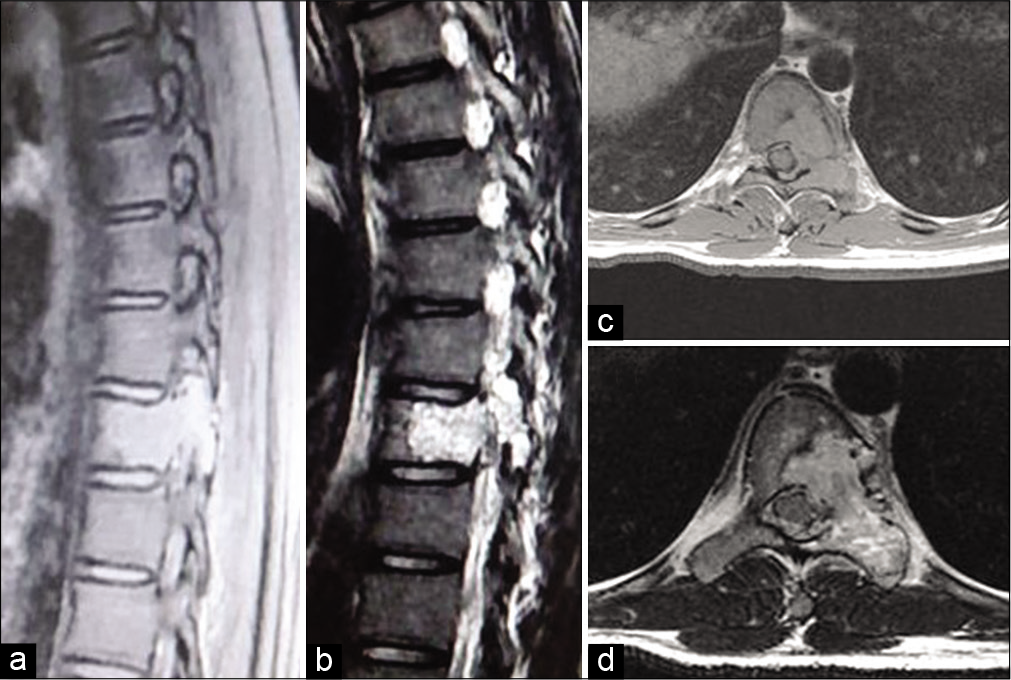
Figure 2:
MRI of the spine. (a and b) T1- and T2-weighted sagittal image revealed a heterogeneous bony cystic mass with internal septation and fluid-fluid levels at the T9 vertebra. (c and d) Axial T1- and T2-weighted images showing large, expansile spinal lesion with multiple fluid levels typical for an aneurysmal bone cyst and cord compression.
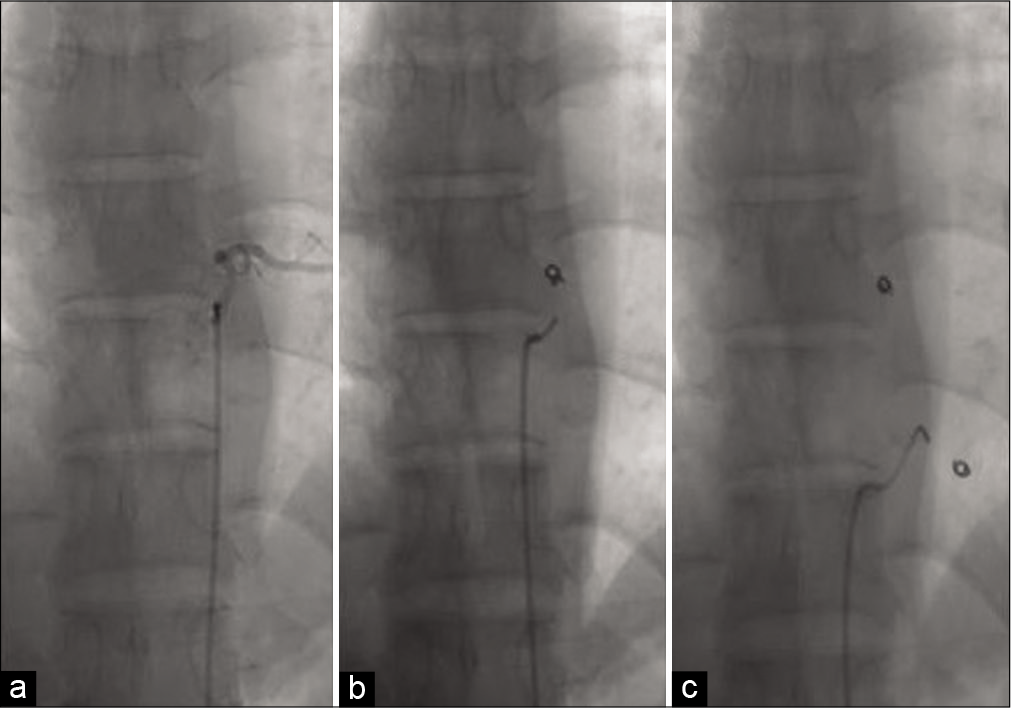
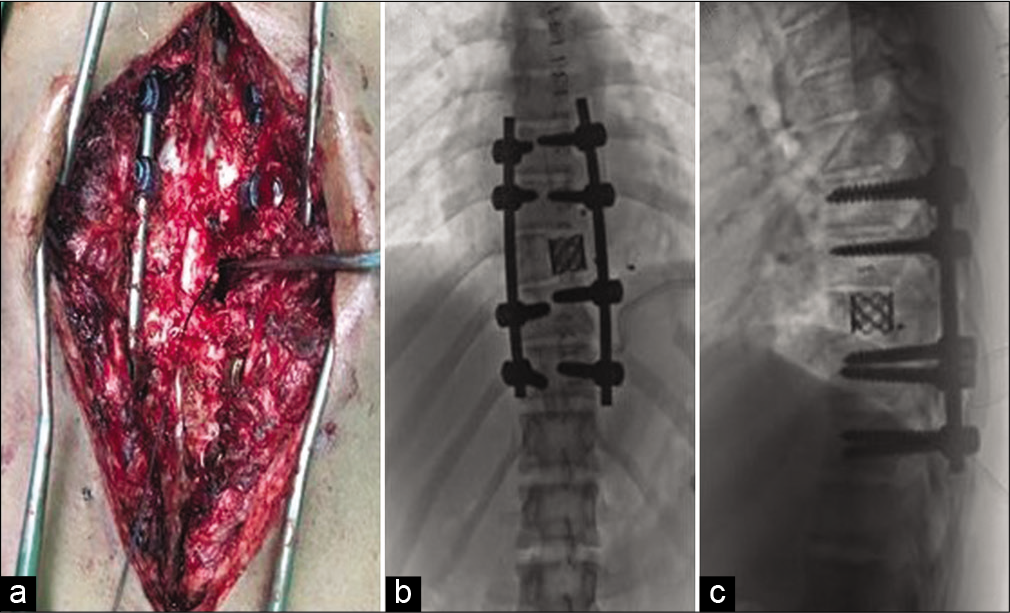
SAE and surgery
The patient had SAE; intercostal feeders were embolized using coils and gel foam [
Pathology/histopathology
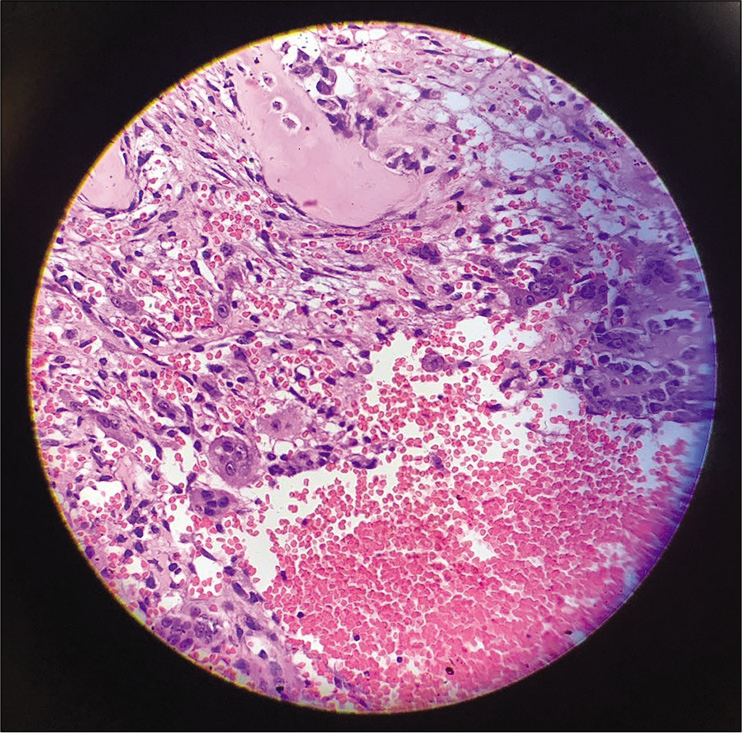
Grossly, the T9 tumor was a gray-red 3–4 cm fleshy mass containing multiple blood-filled cysts. Histopathological examination showed cavernous spaces filled with blood surrounded by fibrous septa with marked cellular proliferation of band fibroblasts, few spindle cells, and scattered giant cells consistent with the diagnosis of an ABC [
Postoperative course
The pain was reduced, and his deficits improved significantly. Within 3 months, he was walking independently and performing routine activities.
Lesion recurrence
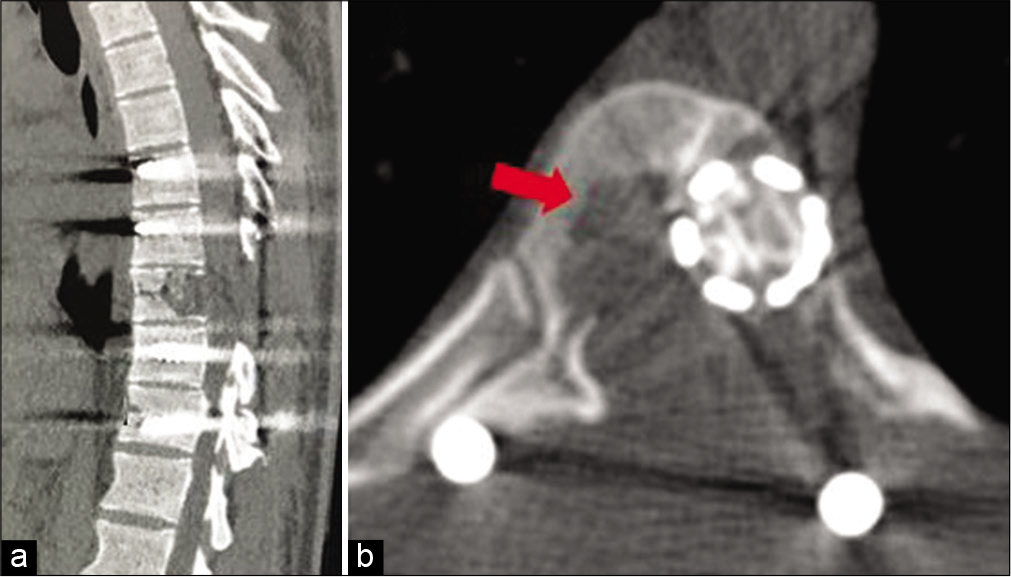
At the 5th postoperative months, he presented with a new right-sided thoracic radiculopathy without any focal neurological deficits. The repeat CT scan showed recurrence of the lesion; it now involved the right side of the T9 vertebral body [
Figure 6:
Computed tomography of the spine at the 5th month. (a) Sagittal section showing recurrence of lytic lesion at T9 vertebra with spinal stabilization. (b) Axial section showing lytic lesion at the right side of T9 vertebral body (marked with arrow) and cage with bone growth at previous left side lesion.
Figure 7:
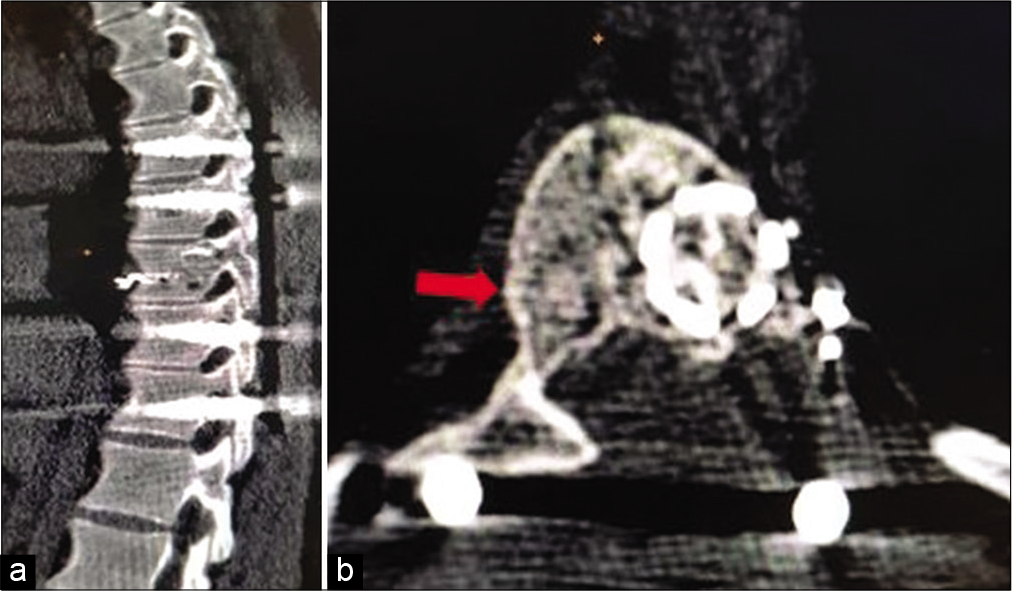
Computed tomography of the spine at end of 2 years. (a) Sagittal section showing complete bone T9 vertebra with intact stabilization and no signs of bony lysis. (b) Axial section showing peripheral sclerotic bone rim formation right side (marked with arrow) and bone formation inside the aneurysmal bone cysts mass.
DISCUSSION
ABCs predominantly occur at the second decade and have a slight preponderance for women.[
Treatment options for primary and recurrent lesions
Tumor recurrences managed with “en bloc” resection, although optimal for lesion control, may not be technically feasible due to – high intraoperative and postoperative morbidity for such extensive resections. Radiation therapy, although very effective, does introduce the risk of radiation-induced sarcoma/myelopathy. SAE may be used preoperatively and/or to treat local recurrences.[
Adjunctive use of denosumab versus bisphosphonate therapy
Denosumab is a human monoclonal antibody that binds the cytokine receptor activator of nuclear factor- kappa B ligand,[
CONCLUSION
We emphasized the importance of surgery for patients with ABC who have focal neurological deficits. However, for those with recurrent lesions without specific neurological findings, SAE with bisphosphonate therapy is effective alternatives to repeated surgical intervention.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Publication of this article was made possible by the James I. and Carolyn R. Ausman Educational Foundation.
Conflicts of interest
There are no conflicts of interest.
References
1. Amendola L, Simonetti L, Simoes CE, Bandiera S, de Iure F, Boriani S. Aneurysmal bone cyst of the mobile spine: The therapeutic role of embolization. Eur Spine J. 2013. 22: 533-41
2. Boriani S, De Iure F, Campanacci L, Gasbarrini A, Bandiera S, Biagini R. Aneurysmal bone cyst of the mobile spine: Report on 41 cases. Spine (Phila Pa 1976). 2001. 26: 27-35
3. Boriani S, Lo SF, Puvanesarajah V, Fisher CG, Varga PP, Rhines LD. Aneurysmal bone cysts of the spine: Treatment options and considerations. J Neurooncol. 2014. 120: 171-8
4. Bulgin D, Irha E, Hodzic E, Hodzic E, Nemec B. Autologous bone marrow derived mononuclear cells combined with β-tricalcium phosphate and absorbable atelocollagen for a treatment of aneurysmal bone cyst of the humerus in child. J Biomater Appl. 2013. 28: 343-53
5. Buraczewski J, Dabska M. Pathogenesis of aneurysmal bone cyst. Relationship between the aneurysmal bone cyst and fibrous dysplasia of bone. Cancer. 1971. 28: 597-604
6. Capanna R, Albisinni U, Picci P, Calderoni P, Campanacci M, Springfield DS. Aneurysmal bone cyst of the spine. J Bone Joint Surg Am. 1985. 67: 527-31
7. Dahlin DC, Unni KK.editors. Bone Tumors. General Aspects and Data on 11,087 Cases. Philadelphia, PA: Lippincott-Raven; 1996. p. 382-90
8. de Kleuver M, van der Heul RO, Veraart BE. Aneurysmal bone cyst of the spine: 31 Cases and the importance of the surgical approach. J Pediatr Orthop B. 1998. 7: 286-92
9. DeRosa GP, Graziano GP, Scott J. Arterial embolization of aneurysmal bone cyst of the lumbar spine. A report of two cases. J Bone Joint Surg. 1990. 72: 777-80
10. Doyle A, Field A, Graydon A. Recurrent aneurysmal bone cyst of the cervical spine in childhood treated with doxycycline injection. Skeletal Radiol. 2015. 44: 609-12
11. Guarnieri G, Vassallo P, Muto M, Muto M. Percutaneous treatment of symptomatic aneurysmal bone cyst of L5 by percutaneous injection of osteoconductive material (Cerament). J Neurointerv Surg. 2014. 6: e43
12. Hay MC, Paterson D, Taylor TK. Aneurysmal bone cyst of the spine. J Bone Joint Surg Br. 1978. 60: 406-11
13. Kieser DC, Mazas S, Cawley DT, Fujishiro T, Tavolaro C, Boissiere L. Bisphosphonate therapy for spinal aneurysmal bone cysts. Eur Spine J. 2018. 27: 851-8
14. Lau AW, Pringle LM, Quick L. TRE17/ubiquitin-specific protease 6 (USP6) oncogene translocated in aneurysmal bone cyst blocks osteoblastic maturation via an autocrine mechanism involving bone morphogenetic protein dysregulation. J Biol Chem. 2010. 285: 37111-20
15. Mahnken AH, Nolte-Ernsting CC, Wildberger JE, Heussen N, Adam G, Wirtz DC. Aneurysmal bone cyst: Value of MR imaging and conventional radiography. Eur Radiol. 2003. 13: 1118-24
16. McDonald P, Letts M, Sutherland G, Unruh H. Aneurysmal bone cyst of the upper thoracic spine. An operative approach through a manubrial sternotomy. Clin Orthop Relat Res. 1992. 279: 127-32
17. Oliveira AM, Chou MM, Perez-Atayde AR, Rosenberg AE. Aneurysmal bone cyst: A neoplasm driven by upregulation of the USP6 oncogene. J Clin Oncol. 2006. 24: e1
18. Rapp TB, Ward JP, Alaia MJ. Aneurysmal bone cyst. J Am Acad Orthop Surg. 2012. 20: 233-41
19. Ruiter DJ, van Rijssel TG, van der Velde EA. Aneurysmal bone cysts: A clinicopathological study of 105 cases. Cancer. 1977. 39: 2231-9
20. Simm PJ, O’Sullivan M, Zacharin MR. Successful treatment of a sacral aneurysmal bone cyst with zoledronic acid. J Pediatr Orthop. 2013. 33: E61-4
21. Skubitz KM, Peltola JC, Santos ER, Cheng EY. Response of aneurysmal bone cyst to denosumab. Spine (Phila Pa 1976). 2015. 40: E1201-4
22. Ye Y, Pringle LM, Lau AW, Riquelme DN, Wang H, Jiang T. TRE17/USP6 oncogene translocated in aneurysmal bone cyst induces matrix metalloproteinase production via activation of NF-kappaB. Oncogene. 2010. 29: 3619-29