Angiomatous meningioma associated with rapidly aggravated peritumoral leptomeningitis: A case report
- Department of Neurosurgery, Suzuka General Hospital, Suzuka, Japan
- Department of Pathology, Suzuka General Hospital, Suzuka, Japan
- Department of Neurosurgery, Mie University Graduate School of Medicine, Tsu, Japan.
Correspondence Address:
Hideki Nakajima, Department of Neurosurgery, Suzuka General Hospital, Suzuka, Japan.
DOI:10.25259/SNI_54_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hideki Nakajima1, Takuro Tsuchiya1, Shigetoshi Shimizu1, Tetsuya Murata2, Hidenori Suzuki3. Angiomatous meningioma associated with rapidly aggravated peritumoral leptomeningitis: A case report. 28-Apr-2023;14:159
How to cite this URL: Hideki Nakajima1, Takuro Tsuchiya1, Shigetoshi Shimizu1, Tetsuya Murata2, Hidenori Suzuki3. Angiomatous meningioma associated with rapidly aggravated peritumoral leptomeningitis: A case report. 28-Apr-2023;14:159. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12292
Abstract
Background: A special type of meningioma is known to have infiltrated inflammatory cells within the tumor, associated with peritumoral inflammation. However, there have been no reports of meningioma with inflammatory response only around the tumor, without inflammatory cells within the tumor itself.
Case Description: A 70-year-old woman presented with transient right hemiparesis due to an extra-axial tumor on the left frontal convexity. The tumor appeared hypointense on T1-weighted magnetic resonance images and hyperintense on T2-weighted images without peritumoral edema, and was homogenously enhanced associated with the peritumoral leptomeningeal enhancement. Cerebrospinal fluid examination showed an increase in the number of inflammatory cells with a predominance of mononuclear cells. During the following 1 month, the tumor size was unchanged, but the peritumoral leptomeningeal enhancement was remarkably enlarged with uncontrolled focal seizures. The tumor was subtotally removed and semisolid substances in the subarachnoid space were biopsied. Pathological examination with immunostaining revealed angiomatous meningioma: the tumor had no inflammatory cell infiltration within it, but was associated with the infiltration of immunoglobulin G4-negative lymphocytes into the border zone between the tumor and the dura mater, as well as numerous neutrophils and fibrinous exudates in the peritumoral subarachnoid space. The tumor removal rapidly improved the leptomeningeal enhancement and inflammatory reactions.
Conclusion: The authors reported the first case of angiomatous meningioma associated with massive peritumoral inflammation without inflammatory infiltrates within the tumor itself.
Keywords: Anti-cyclic citrullinated peptide antibody, Arachnoid enhancement, Inflammation-rich meningioma, Leptomeningitis, Rheumatoid meningitis
INTRODUCTION
Meningiomas derive from meningeal cells of the arachnoid membrane and generally do not affect the subarachnoid space and the brain parenchyma except for those with central nervous system (CNS) World Health Organization (WHO) grade 2 or 3 of moderate or greater malignancy.[
CASE PRESENTATION
A 70-year-old woman with no history presented to a local hospital with transient right hemiparesis 1 month earlier, where computed tomography (CT) of the head revealed an intracranial isodense tumor (30 mm in diameter) with no calcification on the left frontal convexity, being referred to our hospital. On admission, the patient was afebrile and had no neurological deficits. On brain magnetic resonance imaging (MRI), the tumor appeared hypointense on T1-weighted images [
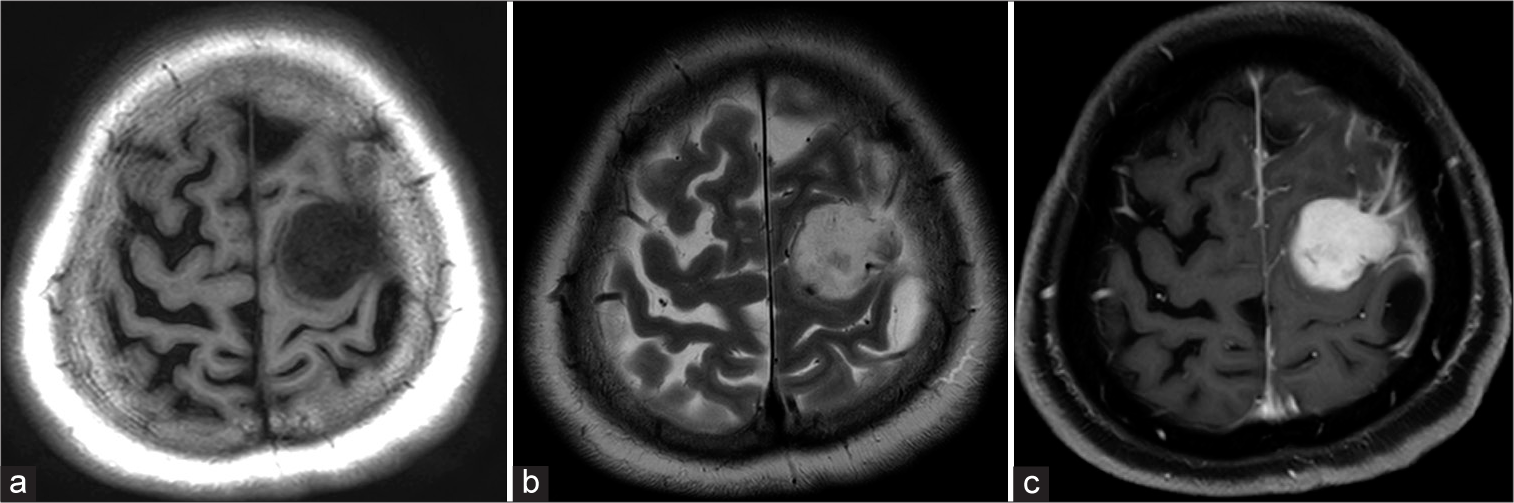
Figure 1:
Magnetic resonance imaging (MRI) on admission (a-c). An extra-axial tumor on the left frontal lobe is hypointense on T1-weighted image (a) and hyperintense without peritumoral edema on T2-weighted image (b). Gadolinium-enhanced T1-weighted image shows a homogenously enhanced tumor with leptomeningeal enhancement around the tumor.
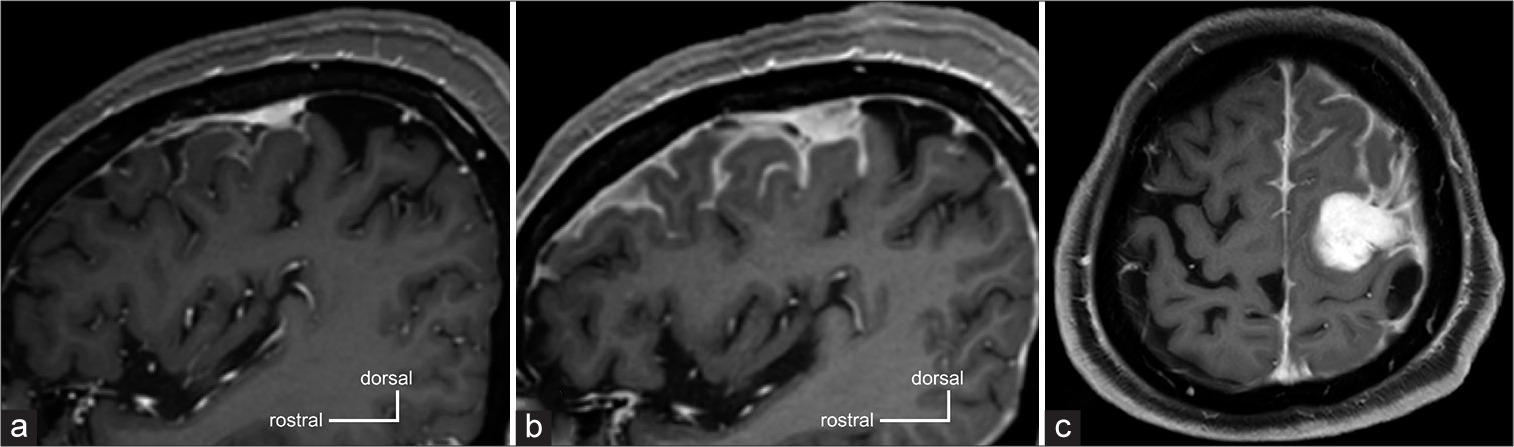
As a differential diagnosis, a solitary dural metastasis of breast cancer could not be ruled out, and close follow-ups were continued. As simple focal seizures of the right upper extremity developed, 1000 mg/day (500mg twice) of levetiracetam was administered. One-month post-admission MRI revealed that the tumor size was unchanged, but peritumoral leptomeningeal gadolinium enhancement was significantly expanded [
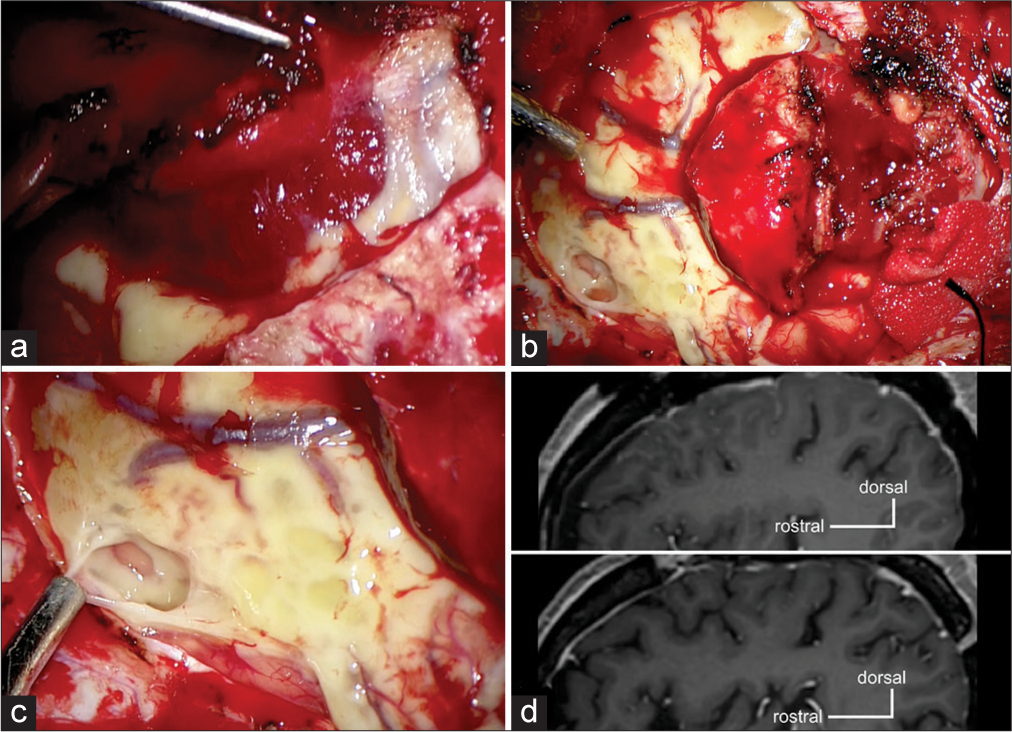
Figure 3:
Intraoperative findings (a-c) and postoperative magnetic resonance imaging (MRI) (d-e). The tumor is soft and reddish (a). The subarachnoid space is broadly filled with yellowish semisolid materials (b), which are biopsied (c). Postoperative MRIs at 1 week (d, upper panel) and 3 years later (d, lower panel) demonstrate a rapid improvement of leptomeningeal enhancement and no recurrence of it.
Pathological findings
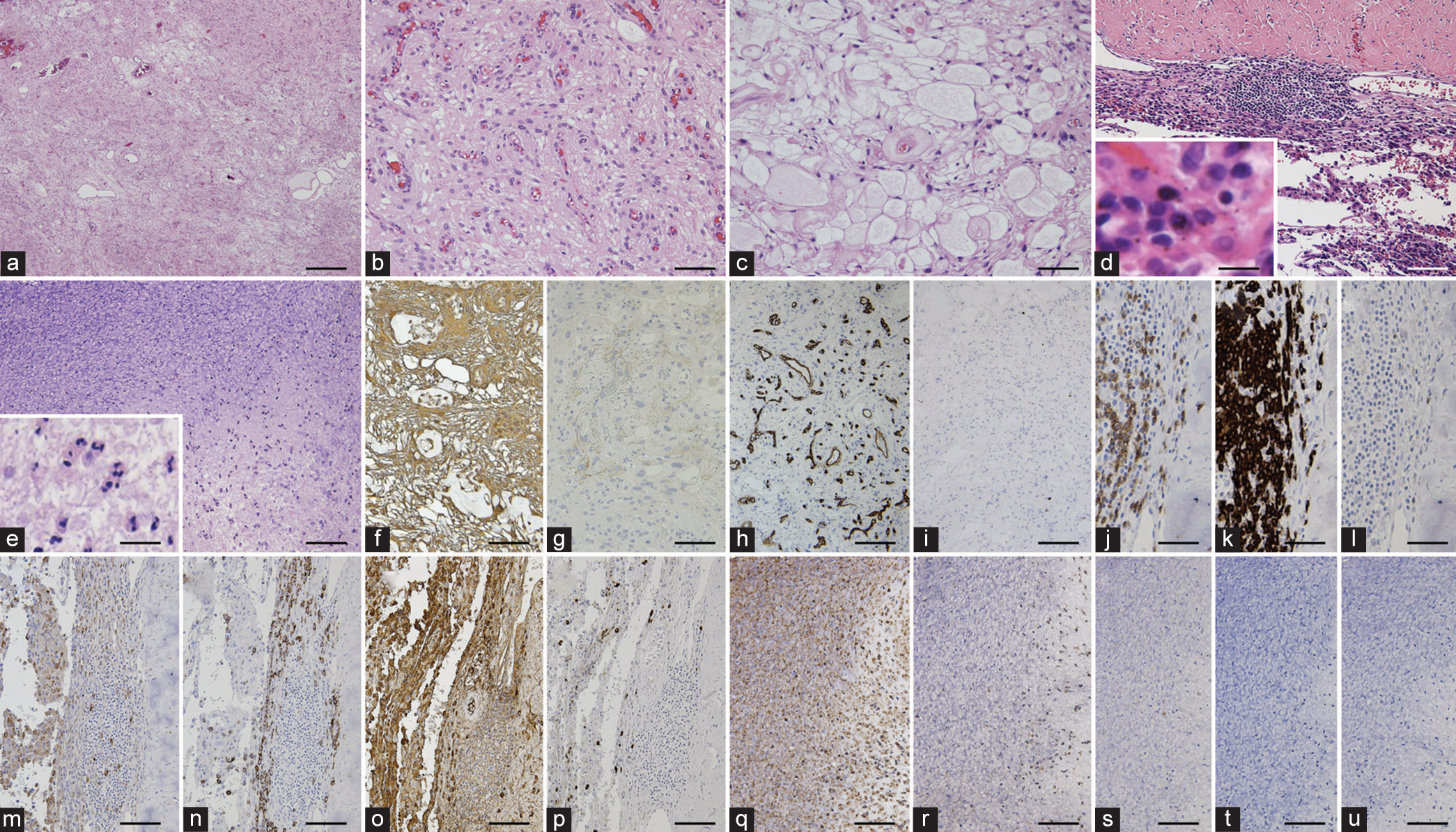
The convexity dural-based tumor [
Figure 4:
Histopathological (hematoxylin and eosin stain; [a-e]) and immunohistochemical (f-u) findings. The convexity dural-based tumor consists of angiomatous components with numerous small to medium-sized vascular channels (a, low-magnification, and b) and microcystic components with microcysts and cobweb-like background (c). Inflammatory infiltrates exist in the border zone between the tumor and the dura mater (d), and mainly consist of lymphocytes (insert of d). The lesion in the subarachnoid space consists of a cluster of neutrophils (insert of e) with fibrinous exudates (e). The tumor cells are reactive for vimentin (f) and slightly reactive for epithelial membrane antigen (g). Vascular endothelial cells were positive for CD34. MIB-1 index is <1%. Inflammatory infiltrates in the border zone between the tumor and the dura mater are reactive for CD3 (j) and CD20 (k), but almost non-reactive for CD15 (l), while those around lymph follicles are positive for CD68 (m) and CD138 (n). The inflammatory cells in the border zone are also broadly reactive for IgG, but those of <10% are reactive for IgG4. In contrast, inflammatory infiltrates in the subarachnoid space are reactive for CD15 (q) and not for CD68 (r), CD45 (s), CD20 (t), and CD138 (u). Bar = 0.5 mm (a), 100 μm (b-g and m-u), 12.5 μm (insert of d), 25 μm (insert of e), 200 μm (h and i), and 50 μm (j-l).
Immunohistochemical study was performed using the Bench Mark ULTRA auto-stainer (Ventana, Tucson, AZ, USA) and the used antibodies were listed in
On the other hand, inflammatory cells infiltrating into the border zone between the tumor and the dura mater were positive for CD3 (T-cell-associated antigen;
DISCUSSION
Meningiomas with inflammation are rare.[
Meningiomas have been reported to elicit inflammatory responses, but inflammatory cells are also known to exacerbate meningiomas.[
Another interesting finding in the present case was the elevation in serum levels of anti-CCP antibody without the symptoms suggestive of RA. Anti-CCP antibodies are RA-specific serum biomarkers and are used for the diagnosis of RA according to the 2010 European League Against Rheumatism criteria.[
CONCLUSION
Inflammation can be closely related to meningiomas, but the pathophysiology remains unclear. The present case is the first report of angiomatous meningioma associated with massive peritumoral inflammatory reactions without inflammatory cell infiltrations within the tumor itself. Further accumulation of similar cases is awaited to elucidate the pathophysiology and the relationships between meningioma and inflammation.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Bathon JM, Moreland LW, DiBartolomeo AG. Inflammatory central nervous system involvement in rheumatoid arthritis. Semin Arthritis Rheum. 1989. 18: 258-66
2. Buerki RA, Horbinski CM, Kruser T, Horowitz PM, James CD, Lukas RV. An overview of meningiomas. Future Oncol. 2018. 14: 2161-77
3. Caputi L, Boncoraglio GB, Bernardi G, Ciusani E, Dantes M, de Liso F. Anti-cyclic citrullinated peptide antibody index in the cerebrospinal fluid for the diagnosis and monitoring of rheumatoid meningitis. Biomedicines. 2022. 10: 2401
4. Cha YJ, Lee SK, Chang JH, Kim SH. Report of a rare case of atypical lymphoplasmacyte-rich meningioma in the tentorium mimicking idiopathic hypertrophic pachymeningitis. Brain Tumor Pathol. 2016. 33: 216-21
5. Du Z, Abedalthagafi M, Aizer AA, McHenry AR, Sun HH, Bray MA. Increased expression of the immune modulatory molecule PD-L1 (CD274) in anaplastic meningioma. Oncotarget. 2015. 6: 4704-16
6. Firdaus M, Gill AS, Andriani R, Cahyanti D, Yunti MR, Faried A. A rare cystic lymphoplasmacyte-rich meningioma: A case report and review of the literature. Surg Neurol Int. 2020. 11: 391
7. Gavrilă BI, Ciofu C, Stoica V. Biomarkers in rheumatoid arthritis, what is new?. J Med Life. 2016. 9: 144-8
8. Goulam-Houssein S, Grenville JL, Mastrocostas K, Munoz DG, Lin A, Bharatha A. IgG4-related intracranial disease. Neuroradiol J. 2019. 32: 29-35
9. Haslund-Vinding J, Møller JR, Ziebell M, Vilhardt F, Mathiesen T. The role of systemic inflammatory cells in meningiomas. Neurosurg Rev. 2022. 45: 1205-15
10. Higashida-Konishi M, Izumi K, Tsukamoto M, Ohya H, Takasugi N, Hama S. Anti-cyclic citrullinated peptide antibody in the cerebrospinal fluid in patients with rheumatoid arthritis who have central nervous system involvement. Clin Rheumatol. 2020. 39: 2441-8
11. Huang G, Wu L, Mei Z, Yao D. Rheumatoid meningitis without a history of rheumatoid arthritis: A case report and literature review. Rheumatol Int. 2023. 43: 1173-82
12. Kuranari Y, Tamura R, Mikami S, Ohara K, Toda M, Yoshida K. Severe headache in a patient with meningioma showing extensive Dural tail correlates with IgG4-positive plasma cells and eosinophils: A case report and review of literature. Surg Neurol Int. 2018. 9: 202
13. Lal A, Dahiya S, Gonzales M, Hiniker A, Prayson R, Kleinschmidt-DeMasters BK. IgG4 overexpression is rare in meningiomas with a prominent inflammatory component: A review of 16 cases. Brain Pathol. 2014. 24: 352-9
14. Lindstorm KM, Cousar JB, Lopes MB. IgG4-related meningeal disease: Clinic-pathological features and proposal for diagnostic criteria. Acta Neuropathol. 2010. 120: 765-76
15. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, FigarellaBranger D. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol. 2021. 23: 1231-51
16. Magaki S, Chang E, Hammond RR, Yang I, Mackenzie IR, Chou BT. Two cases of rheumatoid meningitis. Neuropathology. 2016. 36: 93-102
17. McKenna MC, Vaughan D, Bermingham N, Cronin S. Rheumatoid arthritis presenting as rheumatoid meningitis. BMJ Case Rep. 2019. 12: bcr-2018-226649
18. Najafi M, Goradel NH, Farhood B, Salehi E, Nashtaei MS, Khanlarkhani N. Macrophage polarity in cancer: A review. J Cell Biochem. 2019. 120: 2756-65
19. Nakayama Y, Watanabe M, Suzuki K, Usuda H, Emura I, Toyoshima Y. Lymphoplasmacyte-rich meningioma: A convexity mass with regional enhancement in the adjacent brain parenchyma. Neuropathology. 2012. 32: 174-9
20. Polyzoidis S, Koletsa T, Panagiotidou S, Ashkan K, Theoharides TC. Mast cells in meningiomas and brain inflammation. J Neuroinflammation. 2015. 12: 170
21. Proctor DT, Huang J, Lama S, Albakr A, Van Marle G, Sutherland GR. Tumor-associated macrophage infiltration in meningioma. Neurooncol Adv. 2019. 1: vdz018
22. Rodriguez Alvarez M, Valencia LM, Seidman R, Acharya A, Espina N, Ravindran N. Rheumatoid meningitis and infection in absence of rheumatoid arthritis history: Review of 31 cases. Clin Rheumatol. 2020. 39: 3833-45
23. Shinonaga M, Chang CC, Suzuki N, Sato M, Kuwabara T. Immunohistological evaluation of macrophage infiltrates in brain tumors. Correlation with peritumoral edema. J Neurosurg. 1988. 68: 259-65
24. Son HJ, Yu IK, Kim SM. Lymphoplasmacyte-rich meningioma with atypical angiomatous feature and an increased deposition of IgG4-positive plasma cells: An unusual case report. Int J Surg Pathol. 2018. 26: 93-7
25. Suisa H, Soustiel JF, Grober Y. IgG4-related pachymeningitis masquerading as foramen magnum meningioma: Illustrative case. J Neurosurg Case Lessons. 2021. 2: CASE21398
26. Zhu HD, Xie Q, Gong Y, Mao Y, Zhong P, Hang FP. Lymphoplasmacyte-rich meningioma: Our experience with 19 cases and a systematic literature review. Int J Clin Exp Med. 2013. 6: 504-15