- Department of Neurosurgery, Koyama Memorial Hospital, Kashima, Japan
- Department of Stroke Prevention and Treatment, Tsukuba, Japan
- Department of Neurosurgery, University of Tsukuba, Tsukuba, Japan.
Correspondence Address:
Toshitsugu Terakado, Department of Neurosurgery, Koyama Memorial Hospital, Kashima, Japan.
DOI:10.25259/SNI_170_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Toshitsugu Terakado1, Yuji Matsumaru2, Eiichi Ishikawa3. Anterior cerebral artery dissection for a patient with ipsilateral aplastic or twig-like middle cerebral artery: An illustrative case report. 28-Apr-2023;14:154
How to cite this URL: Toshitsugu Terakado1, Yuji Matsumaru2, Eiichi Ishikawa3. Anterior cerebral artery dissection for a patient with ipsilateral aplastic or twig-like middle cerebral artery: An illustrative case report. 28-Apr-2023;14:154. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12297
Abstract
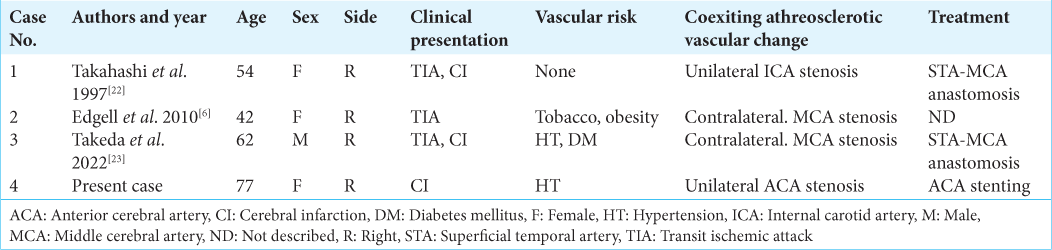
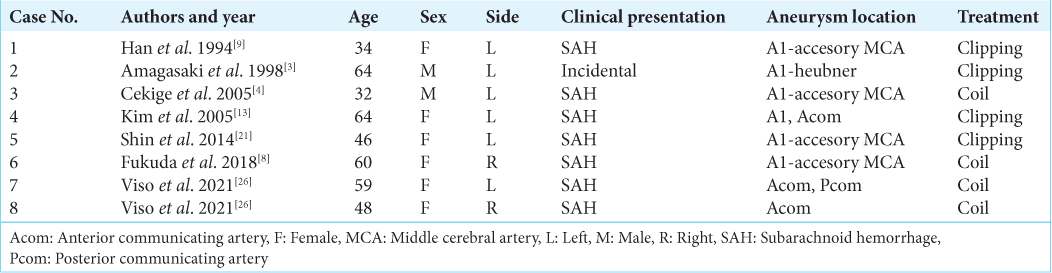
Background: An aplastic or twig-like middle cerebral artery (Ap/T-MCA) is a rare anomaly, which sometimes causes ischemic infarction. Collateral flow from the ipsilateral anterior cerebral artery (ACA) is important for patients with Ap/T-MCA. If ipsilateral ACA stenosis or occlusion occurs, a large infarction with a wider field than the ACA territory could happen. First, mechanical thrombectomy was performed for the right ACA near occlusion caused by arterial dissection with ipsilateral Ap/T-MCA in this case. Second, Wingspan stenting was performed for the right ACA restenosis.
Case Description: A 77-year-old female presented to the hospital with the left hemiparesis. We diagnosed a right ACA infarction caused by right ACA occlusion. Digital subtraction angiography showed right Ap/T-MCA and ipsilateral ACA near occlusion. Thrombectomy was performed, and recanalization was achieved with mild ACA stenosis. The lesion was the dissection due to angiographical finding. Two months after treatment, transient left hemiparesis occurred and right ACA stenosis progressed. Computed tomography perfusion showed hypoperfusion of the right hemisphere. Wingspan stenting was performed from the left internal carotid artery through the anterior communicating artery with an intermediate catheter. The patient was discharged without any neurological deficit.
Conclusion: We reported the first case of a patient who underwent Wingspan stenting for the right ACA dissection with Ap/T-MCA. Short-term follow-up and aggressive intervention should be considered for collateral pathway dissection with Ap/T-MCA because the symptoms can become serious. The patients with Ap/T-MCA should be cautious about the collateral pathway arterial changes in particular ipsilateral ACA due to the increasing hemodynamic stress.
Keywords: Anterior cerebral artery dissection, Aplastic or twig-like middle cerebral artery, Wingspan
INTRODUCTION
An aplastic or twig-like middle cerebral artery (Ap/T-MCA) is a rare occlusive lesion with reticular vessels in the unilateral middle cerebral artery (MCA); its prevalence is very low, at approximately 0.088–1.17%.[
CASE DESCRIPTION
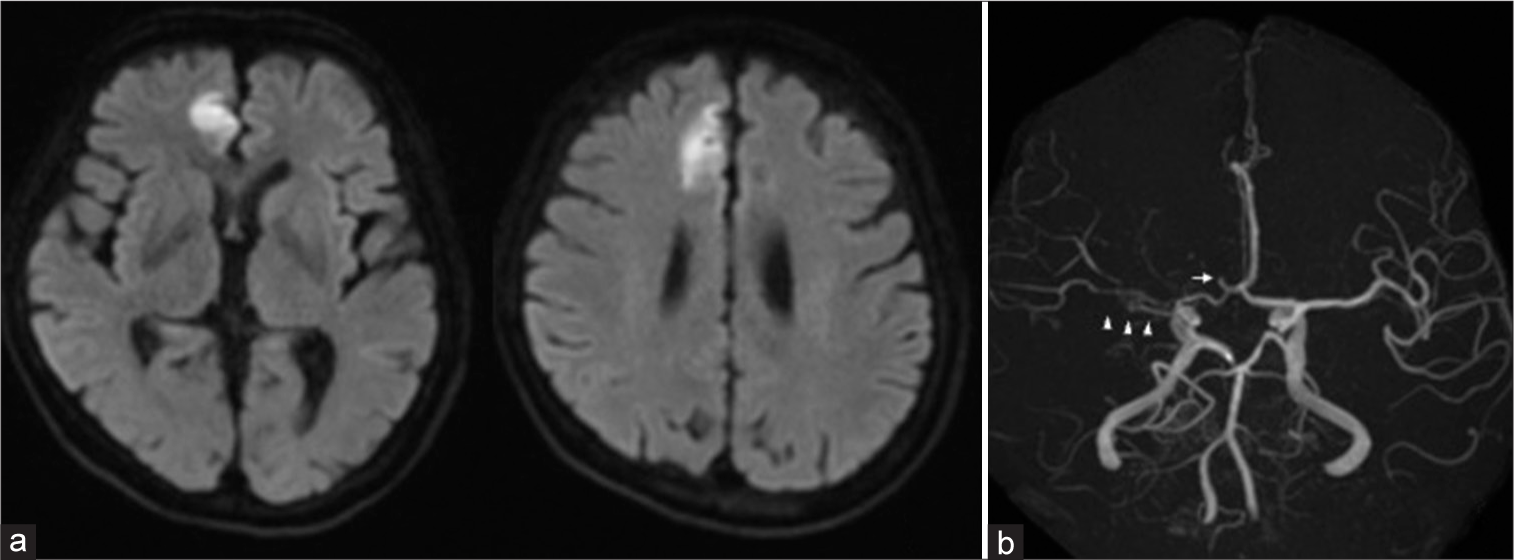
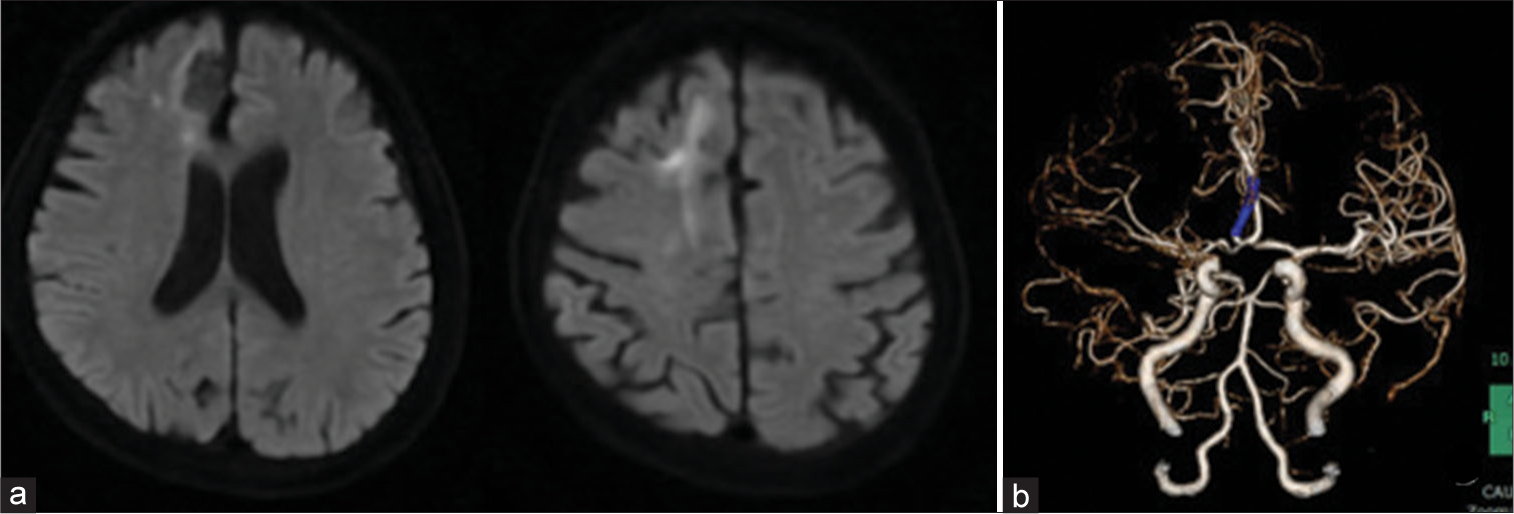
A 77-year-old woman with untreated hypertension and hyperlipidemia presented to our hospital with sudden left hemiparesis. National Institutes of Health Stroke Scale for the patient was 6. Electrocardiogram at admission revealed sinus rhythm and no atrial fibrillation. Brain magnetic resonance imaging (MRI) showed right medial side of the anterior lobe infarction. Brain magnetic resonance angiography (MRA) showed right A2 occlusion and poor visualized right MCA [
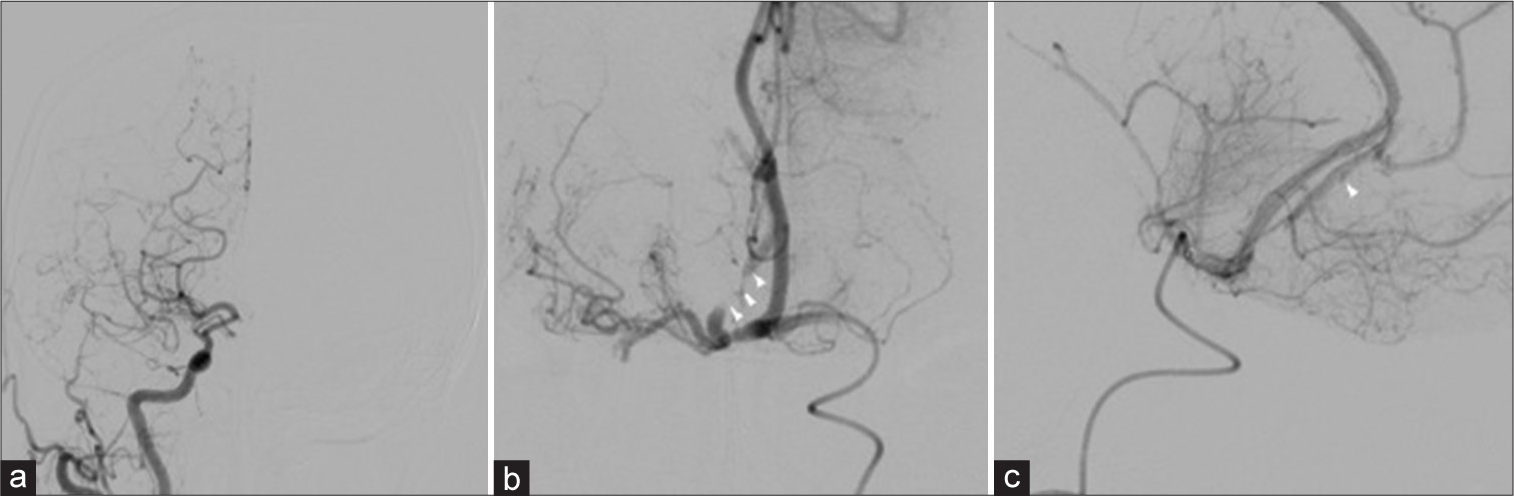
We immediately performed thrombectomy of the right A2. After deploying stent retriever to the right A2 segment, the lesion was identified as a stenotic lesion. Oral administration of loading-dose dual-antiplatelet therapy (200-mg aspirin and 300-mg clopidogrel) was initiated. After the stent was slowly retrieved, recanalization of mild stenosis was accomplished. The lesion was considered dissection due to the presence of dilatation and string sign in retrospect [
Figure 2:
(a) Preoperative DSA of the right common carotid artery showing reticulated vessels developed from the proximal right MCA. Antegrade blood flow from the distal MCA was slow. The right A2 segment was not visualized. (b) Preoperative DSA of the left internal carotid artery showed right A2 near occlusion (white arrowhead). (c) Lateral view of preoperative DSA showed proximal right A2 near occlusion and distal right A2dissection (arrowhead). DSA: Digital subtraction angiography, MCA: Middle cerebral artery.
Figure 3:
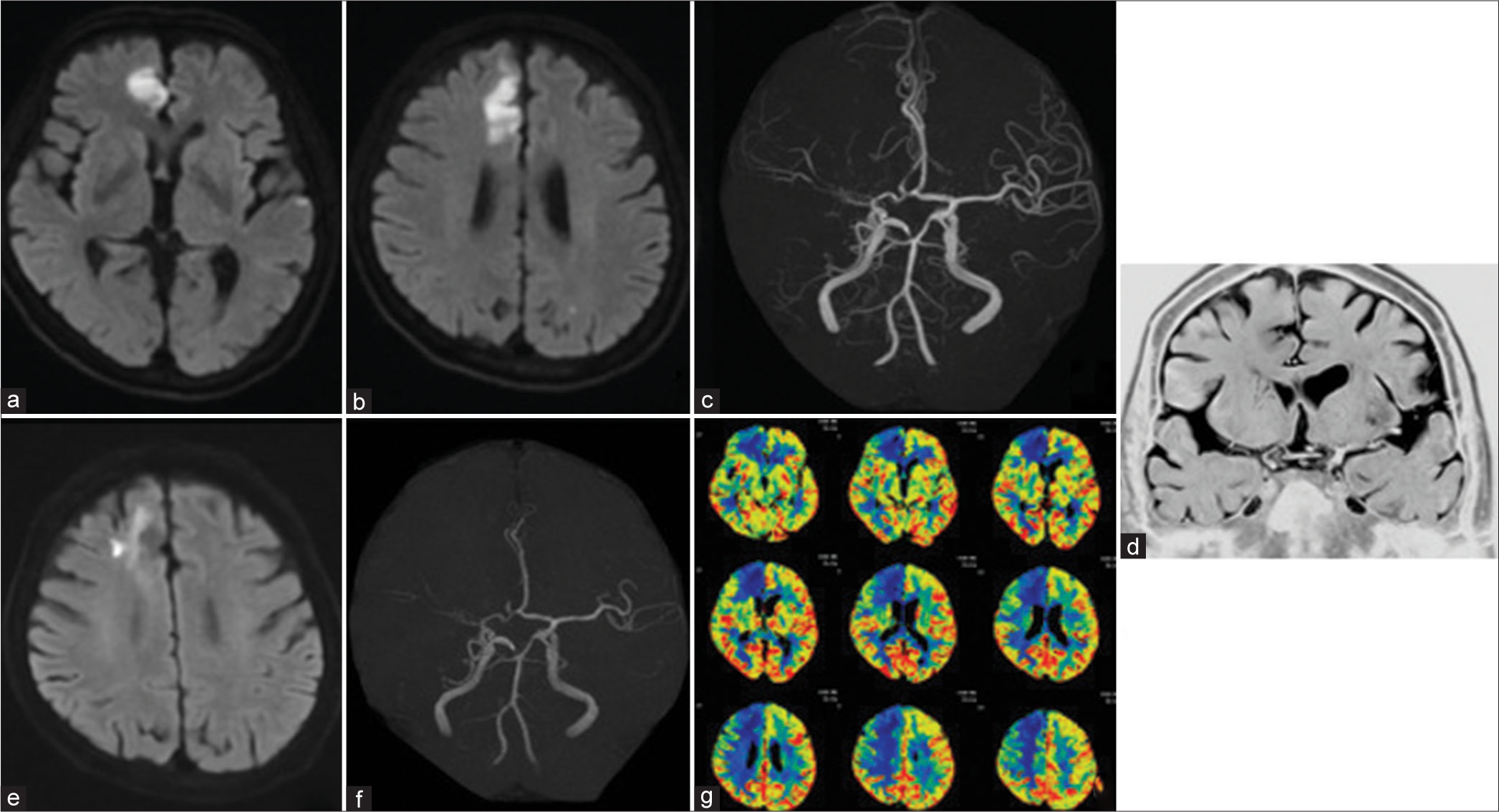
(a and b) DWI showed no newly infarction. (c) Brain MRA showed recanalization of the right A2 segment. (d) Basi-parallel anatomical scanning showed no right M1 form. (e) DWI 2 months later showed an enlarged infarction in the right ACA area. (f) Brain MRA showed right A2 restenosis. (g) Perfusion study of computed tomography scan showed a decrease in the cerebral blood flow at the right ACA and anterior middle cerebral artery. DWI: Diffusion-weighted imaging, MRA: Magnetic resonance angiography, ACA: Anterior cerebral artery.
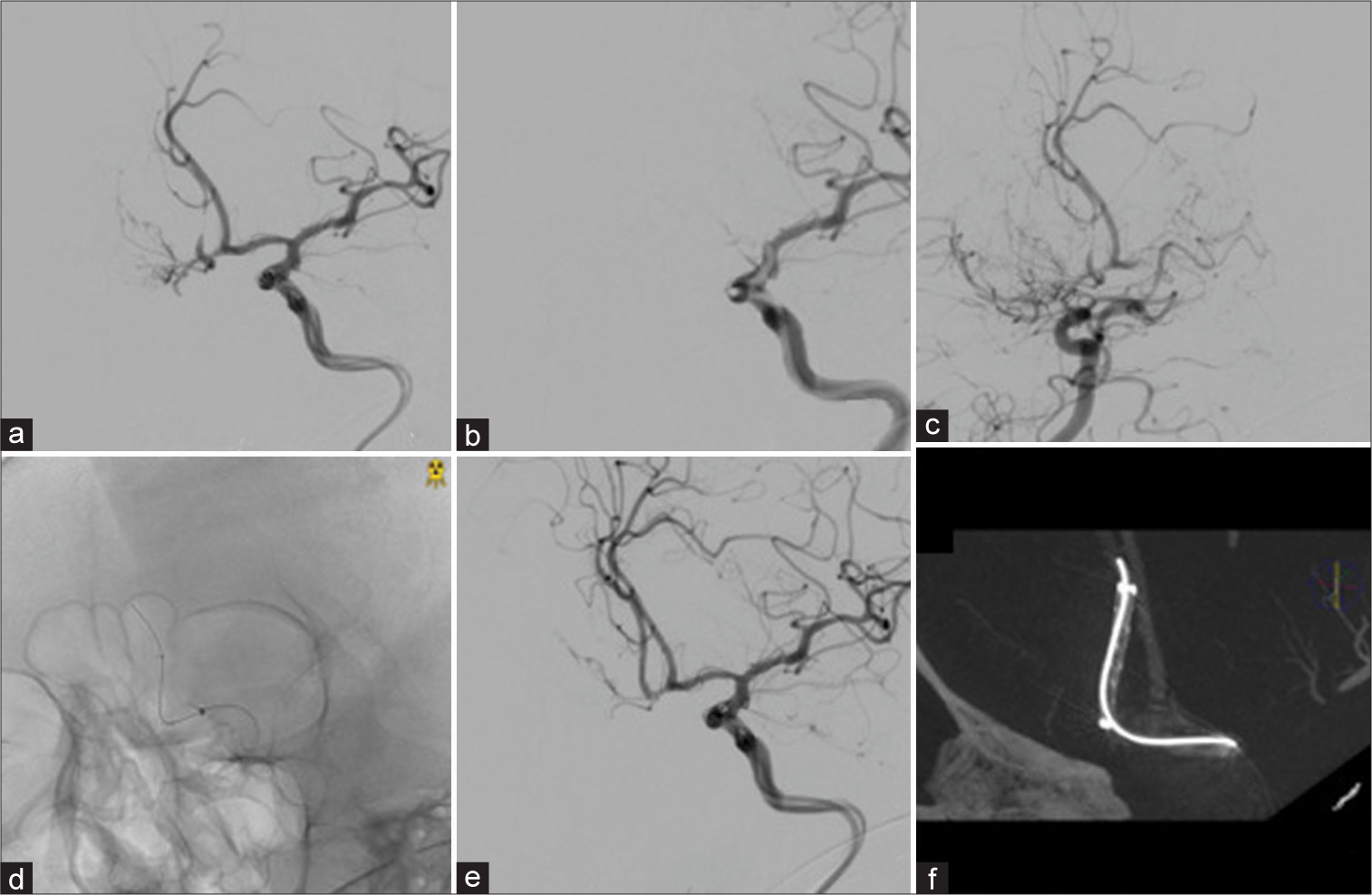
Since perfusion study of the CT scan showed a remarkable decrease in cerebral blood flow (CBF) in the right hemisphere was achieved [
Figure 4:
(a) Right A2 restenosis before the second treatment. (b) Catalyst5 was inserted into the left A1 segment, and antegrade blood flow from the left A1 segment disappeared. (c) The bilateral A2 segment was described from the right A1 segment. (d and e) Catalyst5 was placed in the left A1 segment, and Wingspan was placed in the right A2 segment after stenting. (f) Wingspan was dilated on cone-beam computed tomography.
For this report, consent was obtained from the patient.
DISCUSSION
Ap/T-MCA is a rare vascular anomaly that sometimes causes ischemic infarction.[
In our case, the underlying cause of ACA stenosis could be arterial dissection. We hypothesize that ipsilateral ACA that is the main collateral pathway for the Ap/T-MCA hemisphere is under continuous hemodynamic stress and causes arterial dissection. Other arterial changes of the ipsilateral ACA, including aneurysmal formation, probably due to hemodynamic stress, were seen with Ap/T-MCA [
For treating ACA stenosis or occlusion with ischemic infarction, superficial temporal artery-radial artery graft-A3 bypass or A3–A3 bypass surgery has been reported to be effective.[
Owing to these reasons, stent implantation is considered reasonable for the intracranial artery dissection in ischemic stroke,[
Our statement from this case report has some limitations. First, long-term outcomes are unknown because the patient was only followed up 3 months after treatment. The restenosis rate after Wingspan stenting was reported to be 11.1–17.6%.[
CONCLUSION
We reported the first case of a patient with Ap/T-MCA complicated by ipsilateral A2 dissection who underwent Wingspan stenting. Short-term follow-up and aggressive intervention should be considered for treating collateral pathway dissection with Ap/T-MCA because the symptoms can become serious with time. The patients with Ap/T-MCA need to be cautious about the collateral pathway arterial changes, especially ipsilateral ACA, due to the increasing hemodynamic stress.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Akkan K, Ucar M, Kilic L, Celtikci E, Llgit E, Onal B. Infused or twig-like middle cerebral artery. Eur J Radiol. 2015. 84: 2013-8
2. Alexander MJ, Zauner A, Gupta R, Alshekhlee A, Fraser JF, Toth G. The WOVEN trial: Wingspan one-year vascular events and neurologic outcomes. J Neurointerven Surg. 2021. 13: 307-10
3. Amagasaki K, Yagishita T, Kawataki T, Kase M, Nukui H. Middle cerebral artery aplasia associated with an aneurysm of the proximal anterior cerebral artery. Acta Neurochir (Wein). 1998. 140: 1313-4
4. Cekige HS, Peynircioglu B, Saatci I. Endovascular treatment of an “anterior cerebral artery” aneurysm in a patient with “embryonic unfused middle cerebral artery” anomaly: A case report. Neuroradiology. 2005. 47: 690-4
5. Cho KC, Kim JJ, Jang CK, Hong CK, Joo Y, Kim YB. Rete middle cerebral artery anomalies: A unifying name, case series, and literature review. J Neurosurg. 2018. 131: 453-61
6. Edgell RC, Boulos AS, Haghighi AB, Bernardini GL, Yavagal DR. Middle cerebral artery stenosis associated with moyamoya pattern collateralization. Front Neurol. 2010. 1: 119
7. Feng Z, Duan G, Zhang P, Chen L, Xu Y, Hong B. Enterprise stent for the treatment of symptomatic intracranial atherosclerotic stenosis: An initial experience of 44 patients. BMC Neurol. 2015. 15: 187
8. Fukuda Y, Matsunaga Y, Hirayama K, Yoshimura S, Senkawa T, Sato K. A case of aplastic or twig-like middle cerebral artery associated with a ruptured A1 aneurysm at the origin of the anomalous collateral artery. Jpn J Stroke. 2018. 40: 75-80
9. Han DH, Gwak HS, Chung CK. Aneurysm at the origin of accessory middle cerebral artery associated with middle cerebral artery aplasia: Case report. Surg Neurol. 1994. 42: 388-91
10. Horiuchi T, Ichinose S, Agata M, Ito K, Hongo K. STA-ACA bypass using the ipsilateral free STA graft as an interposition graft and A3-A3 anastomosis for the treatment of bilateral ACA steno-occlusive ischemia. Acta Neurochir (Wien). 2018. 160: 779-82
11. Izumi T, Nishihori M, Imamura H, Iihara K, Sakai N, JRNET Investigators. Endovascular therapy for intracranial artery stenosis: Results from the Japanese Registry of Neuroendovascular Therapy (JR-NET)3. Neurol Med Chir (Tokyo). 2020. 60: 256-63
12. Kim DJ, Kim BM, Suh SH, Kim DI. Self-expanding stent placement for anterior circulation intracranial artery dissection presenting with ischemic symptoms. Neurosurgery. 2015. 76: 158-64
13. Kim MS, Oh CW, Hur JW, Lee JW, Lee HK. Aneurysms located at the proximal anterior cerebral artery and anterior communicating artery associated with middle cerebral artery aplasia: Case report. Surg Neurol. 2005. 64: 534-7
14. Kiyofuji S, Inoue T, Hasegawa H, Tamura A, Saito I. A3-A3 anastomosis and superficial temporal artery-radial artery graft-A3 bypass to treat bilateral ACA steno-occlusive hemodynamic ischemia with cognitive and executive dysfunction: A technical note. Acta Neurochir (Wien). 2014. 156: 2085-93
15. Levy EI, Turk AS, Albuquerque FC, Niemann DB, AagaardKienitz B, Pride L. Wingspan in-stent restenosis and thrombosis: Incidence, clinical presentation, and management. Neurosurgery. 2007. 61: 644-50
16. Liu HM, Lai DM, Tu YK, Wang YH. Aneurysms in twig-like middle cerebral artery. Cerebravasc Dis. 2005. 20: 1-5
17. Lutz T, Monnings P, Ayzenberg I, Lukas C. Twig-like middle cerebral artery: A seldom vessel anomaly of important relevance. Clin Neuroradiol. 2018. 28: 441-3
18. Ma N, Zhang Y, Shuai J, Jiang C, Zhu Q, Chen K. Stenting for symptomatic intracranial arterial stenosis in China: 1-year outcome of a multicentre registry study. Stroke Vasc Neurol. 2018. 3: 176-84
19. Matsunaga Y, Izumo T, Morofuji Y, Horie N, Hayashi K, Matsuo T. Revascularization for aplastic or twiglike middle cerebral artery: A case report. J Stroke Cerebrovasc Dis. 2018. 27: e78-9
20. Seo BS, Lee YS, Lee HG, Lee JH, Ryu KY, Kang DG. Clinical and radiological features of patients with aplastic or twiglike middle cerebral arteries. Neurosurgery. 2012. 70: 1472-80
21. Shin HS, Lee SH, Ryu CW, Koh JS. Flow-related intracranial aneurysms associated with unfused arterial twigs relevant to different vascular anomalies: Embryologic and hemodynamic considerations. Acta Neurochir (Wein). 2014. 156: 1637-46
22. Takahashi M, Fujimoto T, Suzuki R, Asai J, Miyo T, Hokaku H. A case of spontaneous middle cerebral artery occlusion associated with a cerebral aneurysm angiographically disappearing after STA-MCA anastomosis. No Shinkei Geka. 1997. 25: 727-32
23. Takeda H, Yanaka K, Onuma K, Nakamura K, Ishii K, Ishikawa E. Aplastic or twiglike middle cerebral artery with contralateral middle cerebral artery stenosis showing transient ischemic attack: Illustrative case. J Neurosurg Case Lessons. 2022. 3: CASE22121
24. Tsunoda S, Inoue T, Segawa M, Okubo S, Akabane A. Revascularization to the ACA: Effectiveness and variation of the STA-RAG-A3 bonnet bypass. Acta Neurochir (Wien). 2021. 163: 3483-93
25. Uchiyama T, Okamoto H, Koguchi M, Tajima Y, Suzuyam K. A case of aplastic or twig-like middle cerebral artery presenting with an intracranial hemorrhage two years after a transient ischemic attack. No Shinkei Geka. 2016. 44: 143-8
26. Viso R, Lylyk I, Albiña P, Lundquist J, Scrivano E, Lylyk P. Hemorrhagic events associated with unfused or twig-like configuration of the middle cerebral artery: A rare vascular anomaly with clinical relenvance. Interv Neuroradiol. 2021. 27: 285-90
27. Watanabe N, Marushima A, Hino T, Minamimoto S, Sato M, Ito Y. A ruptured aneurysm in aplastic or twig-like middle cerebral artery: A case report with histological investigation. NMC Case Rep J. 2022. 9: 7-12
28. Yamada D, Ishibashi R, Kinosada M, Kurosaki Y, Handa A, Chin M. Aplastic or twig-like middle cerebral artery with short-term ischemia and bleeding. Jpn J Stroke. 2020. 42: 190-5
29. Zhang K, Li TX, Wang ZL, Gao BL, Gu JJ, Gao HL. Factors affecting in-stent restenosis after angioplasty with Enterprise stent for intracranial atherosclerotic diseases. Sci Rep. 2021. 11: 10479