- Department of Surgery, Faculty of Medicine, Vajira Hospital, Navamindradhiraj University, Bangkok, Thailand.
DOI:10.25259/SNI_565_2019
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Somkiat Wongsuriyanan, Kitiporn Sriamornrattanakul. Anterior temporal approach for clipping of ruptured basilar tip aneurysms: Surgical techniques and treatment outcomes. 13-Jun-2020;11:146
How to cite this URL: Somkiat Wongsuriyanan, Kitiporn Sriamornrattanakul. Anterior temporal approach for clipping of ruptured basilar tip aneurysms: Surgical techniques and treatment outcomes. 13-Jun-2020;11:146. Available from: https://surgicalneurologyint.com/surgicalint-articles/10075/
Abstract
Background: Basilar tip (BT) aneurysms are challenging to treat with microsurgical clipping, especially in subarachnoid hemorrhage cases. The anterior temporal approach is one of the surgical approaches for the treatment of aneurysms in this area. The majority of the previous reports on this approach have described unruptured cases. For the ruptured cases assessed in our study, the authors describe the surgical technique, patient characteristics, and surgical outcomes following the use of this technique.
Methods: Fourteen patients with ruptured BT aneurysms who received aneurysm clipping with an anterior temporal approach between December 2015 and August 2019 were retrospectively evaluated. The surgical techniques are described, an illustrative case is shown.
Results: The average patient age was 62.2 years (range: 46–78) for ten women and four men. Nine patients (64.3%) were classified as having a poor grade (World Federation of Neurosurgical Societies Grades 4 and 5) at the first presentation. All of the cases demonstrated complete aneurysm obliteration. Good outcomes (mRS 0 to 2) at 6 months were achieved in 58.3% of the patients and in 77.8% of the patients who had a good Glasgow Coma Score after resuscitation before surgery. Postoperative transient oculomotor nerve palsy and thalamic infarctions were detected in six patients (42.9%) and two patients (14.3%), respectively.
Conclusion: With appropriate case selection, the anterior temporal approach was effective and safe for the clipping of ruptured BT aneurysms.
Keywords: Anterior temporal approach, Basilar bifurcation aneurysm, Basilar tip aneurysm, Ruptured aneurysm
INTRODUCTION
The surgical treatment of basilar tip (BT) or basilar bifurcation aneurysms remains a challenge, especially in situations of subarachnoid hemorrhage. The surgical approach for BT aneurysms by itself is very complicated because the aneurysms are situated in the center of the skull base and are covered by critical structures such as internal carotid arteries (ICAs), posterior communicating arteries, posterior cerebral arteries, and many perforators.[
For BT aneurysm treatment, especially in complex cases or in conditions for which endovascular treatments are unavailable, open surgery for aneurysm clipping is still indicated.[
Several surgical techniques have been described for BT aneurysm clipping. A well-known pterional transsylvian approach by Yasargil et al.[
The anterior temporal approach, which is also called the modified distal transsylvian approach, was developed for approach to the upper basilar artery[
The aims of our study were to evaluate the surgical outcomes and surgical complications and to describe the surgical techniques of the anterior temporal approach for clipping the ruptured BT aneurysms with illustrative cases.
MATERIALS AND METHODS
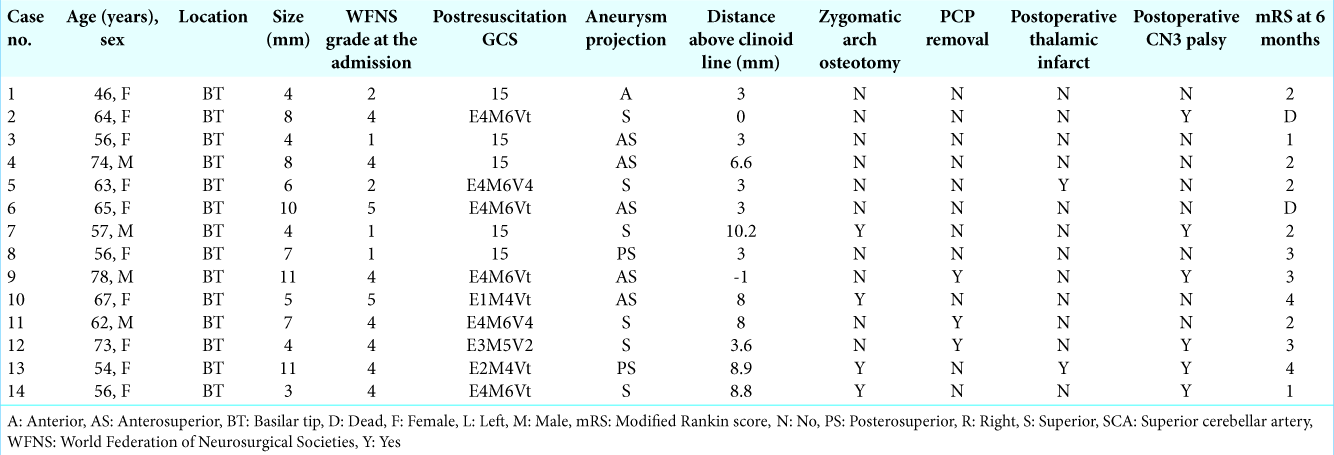
Between December 2015 and August 2019, we retrospectively reviewed patients with ruptured BT aneurysms. During this period of time, the patients needed to undergo open surgery due to the unavailability of endovascular instruments and financial issues. Our selection criteria for open surgery were good grade patients as well as poor grade patients whose consciousness improved after resuscitations, such as intubation and ventriculostomy. The inclusion criteria were as follows: (1) patients with ruptured BT aneurysm; (2) patients in whom the anterior temporal approach was successfully used for aneurysm clipping; and (3) patients with an aneurysm that was not more than 15 mm in size. Cases in which bypass surgery had been performed were excluded from our study. The following preoperative data were collected and analyzed: age, gender, World Federation of Neurosurgical Societies (WFNS) grade (WFNS Grades 1, 2, and 3 are defined as good grade; WFNS Grades 4 and 5 are defined as poor grade), Glasgow Coma Score (GCS) after resuscitation, aneurysm size, aneurysm projection, height of aneurysm neck from the clinoid line (the straight line connecting the superior surface of the anterior clinoid process and the tip of the posterior clinoid process), additional skull base technique (zygomatic arch osteotomy, posterior clinoid process removal, postoperative thalamic infarction, postoperative oculomotor nerve palsy, and Modified Rankin Score (mRS) 6 months after discharge.
Our exclusion criterion for the use of the anterior temporal approach was the lack of space for proximal control at the basilar trunk. In patients with low-positioned BT aneurysms (aneurysm neck was more than 3 mm below the clinoid line), the subtemporal transtentorial approach was selected.
Operative techniques
Since December 2015, our first choice for an approach to the BT aneurysm was the anterior temporal approach. We selected the side of the surgical approach as the side that was more narrowly angled between the aneurysm neck and the posterior cerebellar artery (PCA), the lower position of the P1 segment of the PCA, and the nondominant posterior communicating artery. We also added a zygomatic arch osteotomy for high-positioned aneurysms that were more than 5 mm above the clinoid line. We also performed a posterior clinoidectomy in cases where the aneurysm neck was situated at or below the level of the posterior clinoid process to increase space for proximal control.[
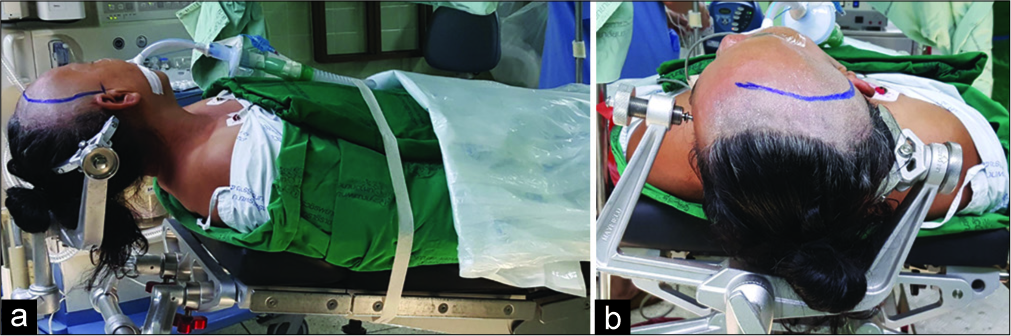
The patient was placed in the supine position with the head of the bed approximately 30 degrees above the level of the heart. The patient’s face was turned to the opposite side approximately 35–45 degrees with the vertex parallel to the floor and the neck extended in the sniffing position [
Figure 1:
Surgical position for the anterior temporal approach. (a) The head of the bed was 30 degrees above the level of the patient’s heart. The vertex of the head was parallel to the floor, and the neck was extended in the sniffing position. (b) The face was turned away from the side of the operation by approximately 35–45 degrees.
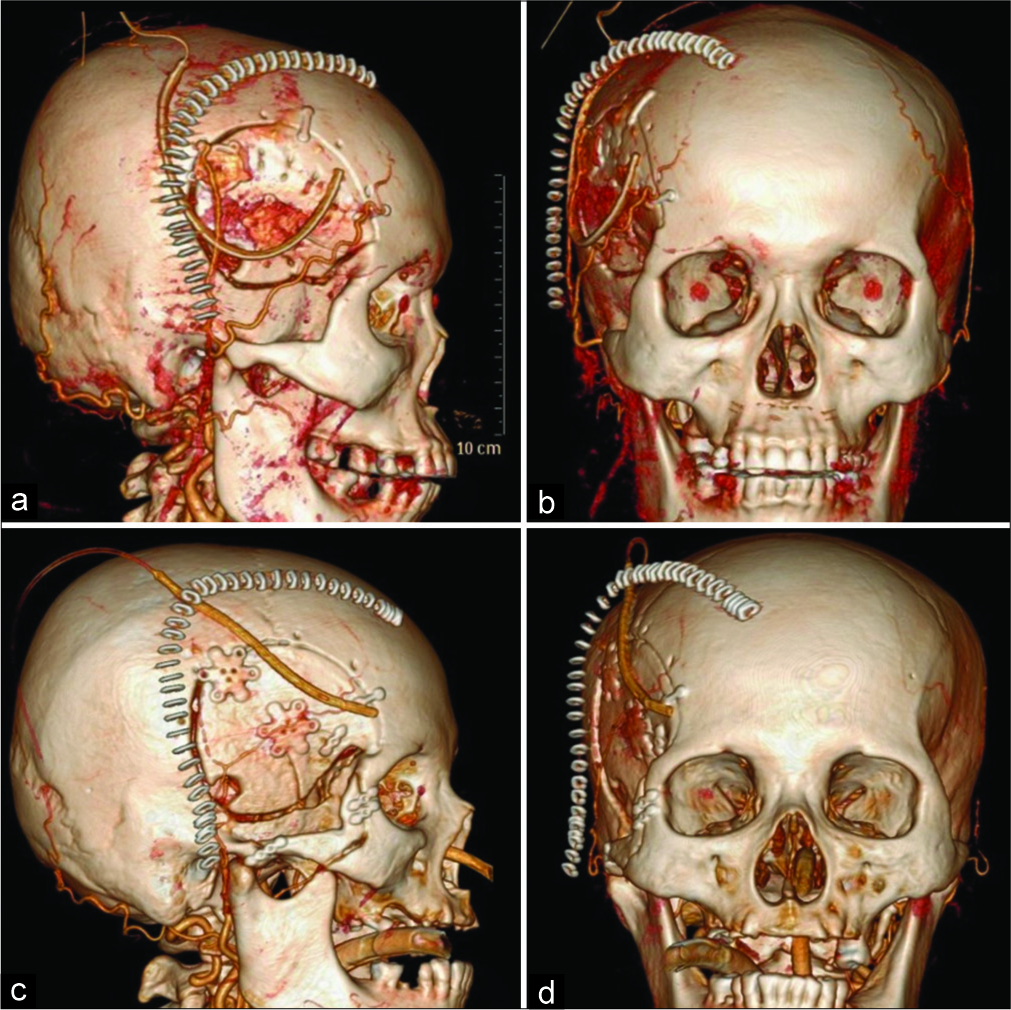
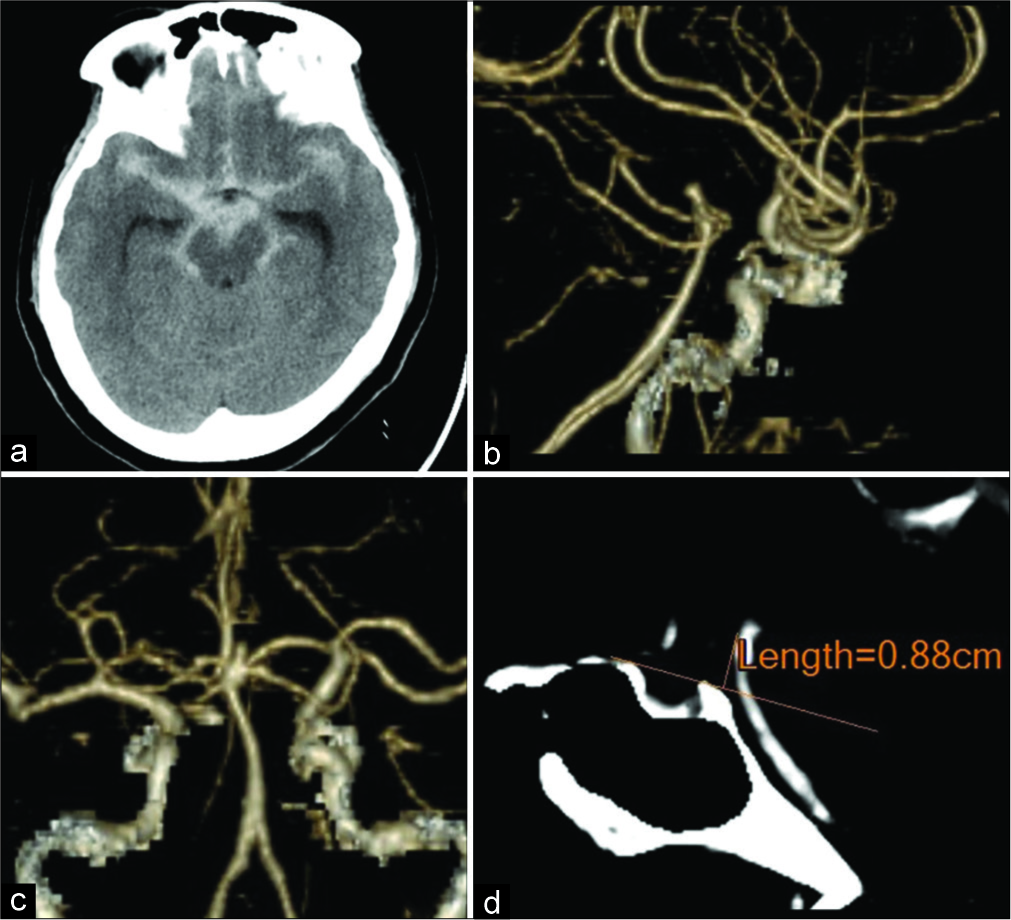
Figure 2:
Postoperative three-dimensional computed tomography angiography. The skin stapler shows a skin incision with a relationship to the superficial temporal artery. The shape of the skull flap is also demonstrated (a and b). Skin incision and frontotemporal craniotomy for the anterior temporal approach (c and d). Skin incision, zygomatic arch osteotomy, and craniotomy were performed for the transzygomatic anterior temporal approach.
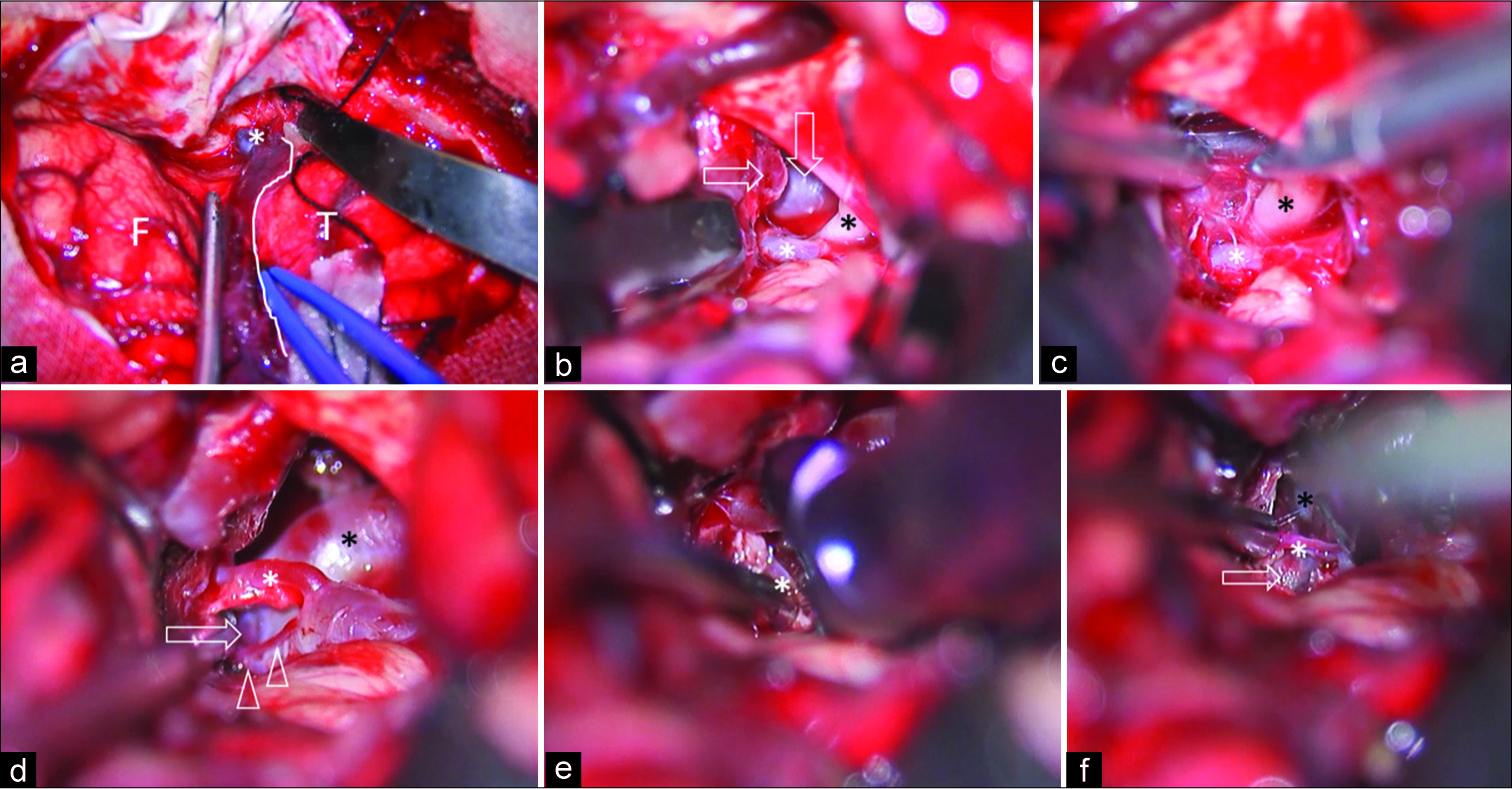
Figure 3:
Intraoperative photographs of the right transzygomatic anterior temporal approach. (a) The dura was opened to expose frontal lobe, temporal lobe, and the Sylvian fissure. The route of Sylvian vein drainage to the base of skull (*) and temporal side of Sylvian vein (white line) were identified. (b) After the Sylvian fissure was widely opened through the temporal side, the Sylvian veins were retracted together with the frontal lobe. The temporal lobe was retracted posteriorly, and then the oculomotor nerve (black asterisk), posterior communicating artery (horizontal arrow), basilar trunk (vertical arrow), and P2 segment of the PCA (white asterisk) were identified. (c) The arachnoid membrane around the nerve (black asterisk) was sharply cut. The P2 segment of the PCA (white asterisk) was located just above the nerve. (d) After the ICA was retracted anteriorly, the basilar trunk (black asterisk), basilar tip aneurysm (arrow), posterior communicating artery (white asterisk), and perforators (arrowhead) were exposed through the retrocarotid space. (e) An aneurysm clip was applied to the aneurysm neck with perforator (asterisk) preservation. (f) After definite aneurysm clipping, the aneurysm dome (arrow), left P1 segment of the PCA (black asterisk), and perforator (white asterisk) were identified. F: Frontal lobe, PCA: Posterior cerebral artery, T: Temporal lobe. ICA: Internal carotid arteries.
Outcomes
The surgical outcomes were evaluated 6 months after discharge with the mRS by direct examinations or telephone interviews. The outcomes, excluding nonsurgical mortalities (such as acute myocardial infarction and sepsis), were divided into overall outcomes and subgroup outcomes of the patients with good GCS (E3-4 M6) after resuscitation (intubation and ventriculostomy). A mRS of 0 to 2 was defined as a good outcome, and an mR S of 3 to 5, and perioperative death were defined as poor outcomes.
RESULTS
A total of 580 cases of open surgery for the treatment of intracranial aneurysms were performed at Vajira Hospital between December 2015 and August 2019. Fourteen cases of ruptured BT aneurysms were successfully treated with clipping through the anterior temporal approach [
Patients’ characteristics
The average patient age was 62.2 years (range; 46–78) with ten women and four men. At the first presentation of subarachnoid hemorrhage, five patients (35.7%) had WFNS Grades 1–3 (good grade), and nine patients (64.3%) had WFNS Grades 4 and 5 (poor grade). After resuscitation, six cases of the poor grade group improved to a good GCS (E3-4 M6) (case no. 2, 4, 6, 9, 11, and 14). Therefore, the number of patients with a good GSC before surgery was 11 (78.6%).
Aneurysm characteristics
The mean aneurysm size and height from the clinoid line were 6.6 mm and 4.9 mm, respectively. A zygomatic arch osteotomy through the anterior temporal approach was performed in four cases (28.6%). A posterior clinoidectomy was needed in four cases (21.4%).
Surgical outcomes and complications of the anterior temporal approach
Computed tomography angiography (CTA) was routinely evaluated in all cases. All cases demonstrated complete aneurysm obliteration. Thalamic infarctions occurred in two cases (14.3%), leading to postoperative mild hemiparesis and impaired consciousness. Postoperative third nerve palsy occurred in six cases (42.9%), and these patients, who were alive, spontaneously and completely recovered within 3 months. One case (case no. 9) demonstrated an ipsilateral anterior choroidal artery injury during clip application, causing severe postoperative hemiparesis. Two patients (cases no. 2 and 6) died from severe sepsis from hospital- acquired pneumonia.
Overall, good outcomes (mRS of 0–2) at 6 months were achieved in seven cases (58.3%) of the living patients (12 cases). For the living patients with good GCS after resuscitation (nine cases), a good outcome at 6 months was accomplished in seven cases (77.8%). Two cases in this group developed poor outcomes due to postoperative hemiparesis from intraoperative anterior choroidal artery injury (case no. 8) and a delayed recovery due to advanced age (case no. 9).
Illustrative case
A 56-year-old female presented with sudden severe headache and altered consciousness [Case No. 14 in
Figure 4:
Case no. 14. Preoperative CT and CTA. (a) Plain CT scan shows diffuse subarachnoid hemorrhage and hydrocephalus. The three-dimensional CTA in the right lateral (b) and posterior views (c) demonstrates a superior projecting aneurysm at the basilar bifurcation. (d) The aneurysm neck was located 8.8 mm above the clinoid line. CTA: Computed tomography angiography, CT: Computed tomography.
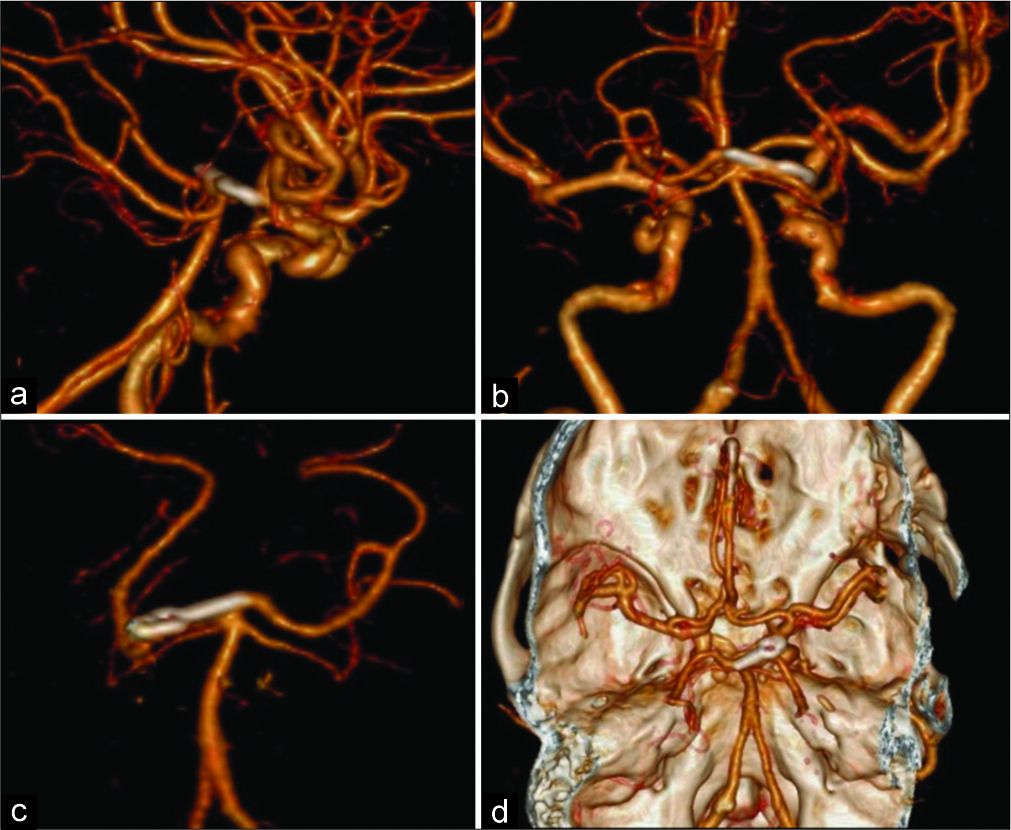
Figure 5:
Case no. 14. Postoperative CTA. The three-dimensional CTA in right lateral (a), posterior views (b), anterior view (c), and superior view with skull base (d) shows the complete obliteration of the aneurysm with the preservation of both posterior cerebral arteries. CTA: Computed tomography angiography.
DISCUSSION
At the present time, most BT aneurysms are treated with endovascular procedures. For endovascular treatment of BT aneurysms, good outcomes were reported in 73–96% of cases, with complete/near complete occlusion rates of 64–89%.[
The famous surgical approaches for BT aneurysms are the subtemporal approach by Drake[
The pterional approach offers an anterolateral direction to the BT aneurysm through the opticocarotid space or retrocarotid space with Sylvian fissure opening. The advantages of this approach are that it provides a good visualization for aneurysm necks located between the midpoint of the sella turcica, which is 1 cm above the posterior clinoid process, and it offers better exposure of the contralateral P1 segment of the PCA with less brain retraction when compared to the subtemporal approach. With this approach, the perforators’ posterior to the aneurysm neck is difficult to see.[
The later-developed surgical approaches to BT aneurysm include a combination of the previously described anterolateral and lateral corridor by posterolateral retraction of the temporal lobe, such as the extended lateral (half- and-half) approach,[
The anterior temporal approach was a modification for anterolateral access to the upper basilar artery and posterior projecting ICA aneurysms. The distinctive point of this approach is the posterolateral retraction of the temporal lobe without sacrificing venous drainage of the Sylvian vein to the base of skull and with less tension to the main trunk of the middle cerebral artery than with the previously described techniques.[
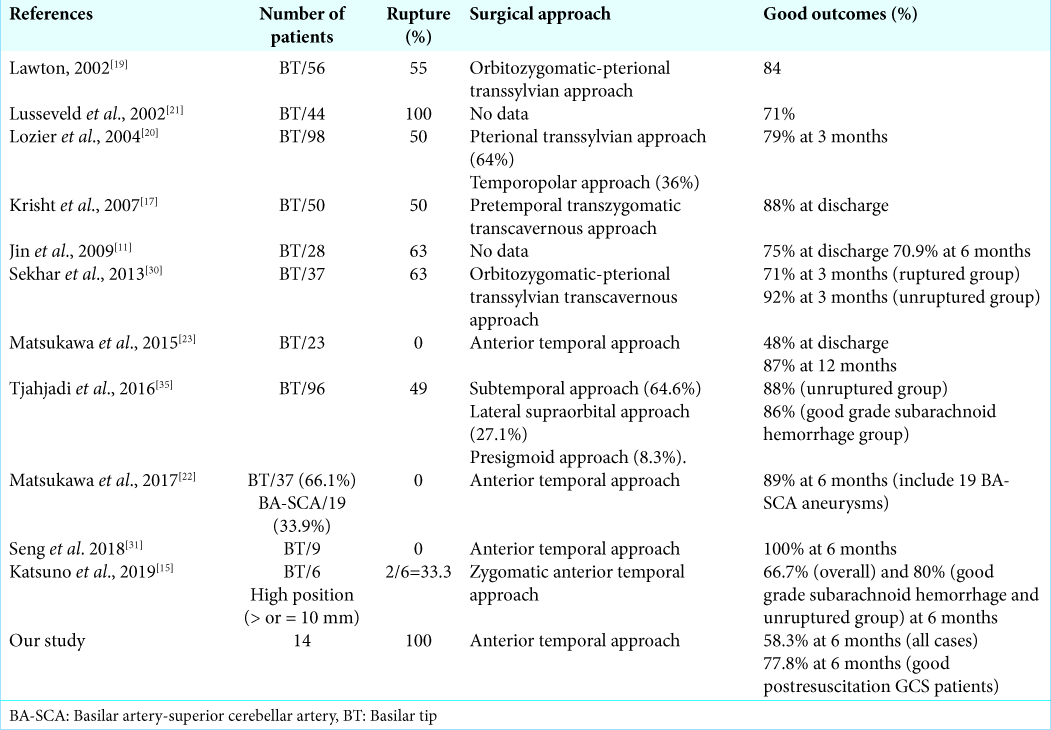
Several published studies of open surgery for BT aneurysms with various preoperative conditions, surgical approaches and postoperative outcomes are shown in
For the anterior temporal approach, which is similar to the approach used in our study but with 100% unruptured cases, Matsukawa et al. reported a study of microsurgical clipping for 23 patients with complex unruptured BT aneurysms in 2015. BT aneurysms with necks >4 mm, posterior projection, retro/subsellar, and dome-to-neck ratios <1.2 were classified as complex BT aneurysms. Eleven patients (48%) had a good outcome (mRS 0 to 1) at discharge, and 87% had a good outcome at the 12-month follow-up. Postoperative oculomotor palsies were detected in 65% of patients. Postoperative diffusion-weighted imaging also revealed ischemia in the posterior thalamoperforating artery distribution in 8.7% of patients and in the anterior choroidal artery distribution in 8.7% of patients.[
In our study, 100% of patients were ruptured cases, in which 64.3% were poor grade at initial presentation and 78.6% were good GSC before surgery. All cases were operated with the anterior temporal approach. At 6 months after operation, the rate of an overall good outcome was 58.3%, which was lower than that in the study by Lusseveld et al. (71%)[
CONCLUSION
With the appropriate case selection, the anterior temporal approach was effective and safe for the clipping of ruptured BT aneurysms.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Andrews RJ, Bringas JR. A review of brain retraction and recommendations for minimizing intraoperative brain injury. Neurosurgery. 1993. 33: 105-264
2. Bendok BR, Getch CC, Parkinson R, O’Shaughnessy BA, Batjer HH. Extended lateral transsylvian approach for basilar bifurcation aneurysms. Neurosurgery. 2004. 55: 174-8
3. De Oliveira E, Tedeschi H, Siqueira MG, Peace DA. The pretemporal approach to the interpeduncular and petroclival regions. Acta Neurochir (Wien). 1995. 136: 204-11
4. Drake CG. Bleeding aneurysms of the basilar artery. Direct surgical management in four cases. J Neurosurg. 1961. 18: 230-8
5. Drake CG. Microsurgical evaluation of the pterional approach to aneurysms of the distal basilar circulation. Neurosurgery. 1978. 3: 140-1
6. Goehre F, Kamiyama H, Noda K, Ota N, Tsuboi T, Miyata S. Technical description of the medial and lateral anterior temporal approach for the treatment of complex proximal posterior cerebral artery aneurysms. World Neurosurg. 2016. 86: 490-6
7. Goehre F, Yanagisawa T, Kamiyama H, Noda K, Ota N, Tsuboi T. Direct microsurgical embolectomy for an acute distal basilar artery occlusion. World Neurosurg. 2016. 86: 497-502
8. Hafez A, Buçard JB, Tanikawa R. Integrated multimaneuver dissection technique of the Sylvian fissure: Operative nuances. Oper Neurosurg (Hagerstown). 2017. 13: 702-10
9. Henkes H, Fischer S, Mariushi W, Weber W, Liebig T, Miloslavski E. Angiographic and clinical results in 316 coil-treated basilar artery bifurcation aneurysms. J Neurosurg. 2005. 103: 990-9
10. Heros RC, Lee SH. The combined pterional/anterior temporal approach for aneurysms of the upper basilar complex: Technical report. Neurosurgery. 1993. 33: 244-50
11. Jin SC, Ahn JS, Kwun BD, Kwon DH. Analysis of clinical and radiological outcomes in microsurgical and endovascular treatment of basilar apex aneurysms. J Korean Neurosurg Soc. 2009. 45: 224-30
12. Jin SC, Park ES, Kwon do H, Ahn JS, Kwun BD, Kim CJ. Endovascular and microsurgical treatment of superior cerebellar artery aneurysms. J Cerebrovasc Endovasc Neurosurg. 2012. 14: 29-36
13. Katsuno M, Tanikawa R, Izumi N, Hashimoto M. A modified anterior temporal approach for low-position aneurysms of the upper basilar complex. Surg Neurol Int. 2015. 6: 10-
14. Katsuno M, Tanikawa R, Miyazaki T, Ota N, Noda K, Izumi N. The limits and countermeasures of the anterior temporal approach for unruptured upper basilar artery aneurysms. No Shinkei Geka. 2013. 41: 311-8
15. Katsuno M, Tanikawa R. Zygomatic anterior temporal approach for high-position upper basilar aneurysm. Neurol Med Chir (Tokyo). 2019. 59: 430-5
16. Krisht AF, Kadri PA. Surgical clipping of complex basilar apex aneurysms: A strategy for successful outcome using the pretemporal transzygomatic transcavernous approach. Neurosurgery. 2005. 56: 261-73
17. Krisht AF, Krayenbühl N, Sercl D, Bikmaz K, Kadri PA. Results of microsurgical clipping of 50 high complexity basilar apex aneurysms. Neurosurgery. 2007. 60: 242-50
18. Kulcsar Z, Ernemann U, Wetzel SG, Bock A, Goericke S, Panagiotopoulos V. High-profile flow diverter (silk) implantation in the basilar artery: Efficacy in the treatment of aneurysms and the role of the perforators. Stroke. 2010. 41: 1690-6
19. Lawton MT. Basilar apex aneurysms: Surgical results and perspectives from an initial experience. Neurosurgery. 2002. 50: 1-8
20. Lozier AP, Kim GH, Sciacca RR, Connolly ES, Solomon RA. Microsurgical treatment of basilar apex aneurysms: Perioperative and long-term clinical outcome. Neurosurgery. 2004. 54: 286-96
21. Lusseveld E, Brilstra EH, Nijssen PC, Van Rooij WJ, Sluzewski M, Tulleken CA. Endovascular coiling versus neurosurgical clipping in patients with a ruptured basilar tip aneurysm. J Neurol Neurosurg Psychiatry. 2002. 73: 591-3
22. Matsukawa H, Kamiyama H, Miyazaki T, Kinoshita Y, Tsuboi T, Noda K. Surgical treatment of unruptured distal basilar artery aneurysm: Durability and risk factors for neurological worsening. Acta Neurochir (Wien). 2017. 159: 1633-42
23. Matsukawa H, Tanikawa R, Kamiyama H, Tsuboi T, Noda K, Ota N. Localization in the interpeduncular cistern as risk factors for the thalamoperforators’ ischemia, poor outcome, and oculomotor nerve palsy in patients with complex unruptured basilar apex aneurysm treated with neck clipping. World Neurosurg. 2015. 84: 465-82
24. Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised trial. Lancet. 2002. 360: 1267-74
25. Nakov VS, Spiriev TY, Todorov IT, Simeonov P. Technical nuances of subtemporal approach for the treatment of basilar tip aneurysm. Surg Neurol Int. 2017. 8: 15-
26. Pandey AS, Koebbe C, Rosenwasser RH, Veznedaroglu E. Endovascular coil embolization of ruptured and unruptured posterior circulation aneurysms: Review of a 10-year experience. Neurosurgery. 2007. 60: 626-36
27. Peluso JP, Van Rooij WJ, Sluzewski M, Beute GN. Coiling of basilar tip aneurysms: Results in 154 consecutive patients with emphasis on recurrent haemorrhage and re-treatment during mid-and long-term follow-up. J Neurol Neurosurg Psychiatry. 2008. 79: 706-11
28. Post N, Russell SM, Jafar JJ. Role of uncal resection in optimizing transsylvian access to the basilar apex: Cadaveric investigation and preliminary clinical experience in eight patients. Neurosurgery. 2005. 56: 274-80
29. Sano K. Temporo-polar approach to aneurysms of the basilar artery at and around the distal bifurcation: Technical note. Neurol Res. 1980. 2: 361-7
30. Sekhar LN, Tariq F, Morton RP, Ghodke B, Hallam DK, Barber J. Basilar tip aneurysms: A microsurgical and endovascular contemporary series of 100 patients. Neurosurgery. 2013. 72: 284-98
31. Seng LB, Yamada Y, Rajagopal N, Mohammad AA, Teranishi T, Miyatani K. Multimodality techniques in microsurgical clipping as the gold standard treatment in the management of basilar tip aneurysm: A case series. Asian J Neurosurg. 2018. 13: 1148-57
32. Spetzler RF, McDougall CG, Zabramski JM, Albuquerque FC, Hills NK, Russin JJ. The barrow ruptured aneurysm trial: 6-year results. J Neurosurg. 2015. 123: 609-17
33. Sriamornrattanakul K, Wongsuriyanan S, Akharathammachote N. Anterior Temporal approach for clipping of upper basilar artery aneurysms: Surgical techniques and treatment outcomes. World Neurosurg. 2019. 131: e530-42
34. Takeuchi S, Tanikawa R, Tsuboi T, Noda K, Oda J, Miyata S. Superficial temporal artery to proximal posterior cerebral artery bypass through the anterior temporal approach. Surg Neurol Int. 2015. 6: 95-
35. Tjahjadi M, Kivelev J, Serrone JC, Maekawa H, Kerro O, Jahromi BR. Factors determining surgical approaches to basilar bifurcation aneurysms and its surgical outcomes. Neurosurgery. 2016. 78: 181-91
36. Tjahjadi M, Serrone J, Hernesniemi J. Should we still consider clips for basilar apex aneurysms? A critical appraisal of the literature. Surg Neurol Int. 2018. 9: 44-
37. Yamamoto J. Clipping of posteriorly projecting large posterior communicating aneurysm via transsylvian anterior temporal approach. Neurosurg Focus. 2015. 39: V15-
38. Yasargil MG, Antic J, Laciga R, Jain KK, Hodosh RM, Smith RD. Microsurgical pterional approach to aneurysms of the basilar bifurcation. Surg Neurol. 1976. 6: 83-91