- Department of Neurosurgery, Hospital Civil de Guadalajara Dr. Juan I Menchaca, Guadalajara, Jalisco, Mexico
- Department of Internal Medicine, Hospital Civil de Guadalajara Dr. Juan I Menchaca, Guadalajara, Jalisco, Mexico
- Department of Pathology, Hospital Civil de Guadalajara Dr. Juan I Menchaca, Guadalajara, Jalisco, Mexico
- Department of Neuroradiology, Hospital Civil de Guadalajara Dr. Juan I Menchaca, Guadalajara, Jalisco, Mexico
Correspondence Address:
Sergio V. Esparza-Gutiérrez
Department of Neuroradiology, Hospital Civil de Guadalajara Dr. Juan I Menchaca, Guadalajara, Jalisco, Mexico
DOI:10.4103/2152-7806.195230
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Adrián Santana-Ramírez, Sergio V. Esparza-Gutiérrez, Pedro Avila-Rodríguez, J. Eugenio Jiménez-Gómez, Ezequiel Vélez-Gómez, David Bañuelos-Gallo. Aspergillosis of the central nervous system in a previously healthy patient that simulated Creutzfeldt–Jakob disease. 05-Dec-2016;7:
How to cite this URL: Adrián Santana-Ramírez, Sergio V. Esparza-Gutiérrez, Pedro Avila-Rodríguez, J. Eugenio Jiménez-Gómez, Ezequiel Vélez-Gómez, David Bañuelos-Gallo. Aspergillosis of the central nervous system in a previously healthy patient that simulated Creutzfeldt–Jakob disease. 05-Dec-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/aspergillosis-of-the-central-nervous-system-in-a-previously-healthy-patient-that-simulated-creutzfeldt-jakob-disease/
Abstract
Background:The presence of Aspergillus in the central nervous system (CNS) is rare in immunocompetent patients but not in immunocompromised patients who may have a more common infection. This article describes a case of an adult immunocompetent patient with a diagnosis of cerebral aspergillosis and with a clinical process of rapidly progressive dementia which simulated a Creutzfeldt–Jakob syndrome.
Case Description:A 34-year-old adult was previously healthy and had no medical history of any significance. The patient had suffered only facial trauma 8 months before admission. One month prior to admission, he showed rapidly progressing changes in his behavior and higher mental functions. He was admitted to the emergency room with an occipital headache with 2 months of history. By the time he arrived, he suffered from total disability and was prostrate. He was diagnosed with meningeal and demential syndrome in the process of being studied. After starting the diagnostic approach by investigating cerebrospinal fluid, a magnetic resonance of the skull, an electroencephalogram, a brain biopsy was indicated. The histopathological study reported the presence of the hyphae characteristics of Aspergillus. The patient died 7 days after the diagnosis.
Conclusion:Cerebral aspergillosis is a common aggressive disease in immunosuppressed patients. However, the disease is rare in individuals with respected immunity and in individuals with neurological impairment and a rapid and progressive deterioration of mental functions. The suspected diagnosis should always be considered given its poor prognosis and the encouraging efficacy of antifungal treatment administered in a timely manner.
Keywords: Cerebral aspergillosis, Creutzfeldt-Jakob, dementia syndrome, meningeal syndrome, spongiform encephalopathy, upper motor neuron syndrome
INTRODUCTION
The presence of Aspergillus in the central nervous system (CNS) is rare in immunocompetent patients but not in immunocompromised patients who may have a more common infection. The prognosis in both cases turns out to be poor in the short-term, with a high mortality, reaching even 100% of untreated cases.
This article describes a case of a young adult patient with respected immunity. The patient has a recent history of rapidly progressive dementia, this characterized in the findings of paraclinical studies with Creutzfeldt–Jakob Syndrome (CJS). A diagnostic approach using a histopathological examination of a brain biopsy indicates the presence of cerebral aspergillosis.
CASE DESCRIPTION
This case report is of a 34-year old man who was married, Catholic, and a construction worker. He had primary school education with no history of previous hospitalizations, surgeries, transfusions, allergies, or known pathologies. He referred only to head trauma suffered 8 months ago by way of physical assault. The assault caused a nose-bone fracture with a nasal septum deviation to the right, but with neither purulent material nor a rinusinusal infectious process. He had only mild, intermittent, and sporadic obstructive dyspnea after the incident. The patient smoked 20 cigarettes a day for 21 years, from 13 to 34 years of age. Up to 5 months prior to his admission, he also suffered from alcoholism for 11 years, drinking 1000 ml of alcohol every week.
He was admitted to the hospital with an occipital headache which he had endured for 2 months. For over 1 and a half months, he had also experienced behavioral disorders and a disruption of higher mental functions. He had retrograde and anterograde amnesia with both echolalia and the presence of visual hallucinations. A private physician initially treated him with diazepam, pentoxifylline, diclofenac, and phenytoin, all with standard doses but with no improvement. Three weeks prior to admission, the patient experienced acalculia and multiple apraxia that did not allow the patient to perform ordinary work and other daily activities such as bathing, and dressing. At this point, the patient required the support of a family member. The individual was evaluated by a second physician who prescribed amitriptyline, bromazepam, B complex, and additional phenytoin. Five days later, the patient developed ataxic gait and was evaluated at a regional hospital where he was given treatment for poisoning (benzodiazepines with flumazenil and a gastric lavage with activated carbon). One week prior to admission, the man quickly and progressively lost the ability to hold himself up. He suffered from psychomotor agitation and myoclonic movements. The patient had a spastic tonicity of his limbs, associated with loud sounds and light. He demonstrated an altered state of consciousness with episodes progressively alternating between a soporose state and an awakened state. He demonstrated akinetic mutism (an indifference to the environment).
He was moved to the Red Cross Hospital where he was given empiric treatment for neuroinfection because data showed a neck stiffness, with a bilateral Babinski, a positive Brudziski, and a negative Kerning and substitutes. Antibiotic therapy was initiated based on a cefotaxime and metronidazole treatment. After failing to show improvement within 4 days, the individual was transferred to our unit where he remained for a period of 25 days and where the aforementioned symptoms continued.
On admission, the patient was received with vital signs within a normal range for his age. He was bedridden and unable to hold himself up. The individual was craniofacial, had intermittent drooling and nostrils without fistula nor the presence of discharge, only the septum veering to the right. The patient showed neck stiffness, without jugular venous engorgement. There were carotid pulses present and no lymphadenopathy at any level of body anatomy was detected. There were no cardiopulmonary or abdomen issues. Other elements of a general physical examination did not show evidence of disease. The patient was neurologically uncooperative and sluggish with alternated isolated episodes of psychomotor agitation. He registered 9 points on the Glasgow Coma Scale (GCS), with the following results: An ocular response to pain (2), localized pain (5), and emission of sounds (2). The patient demonstrated indifference to the environment, was aphasic, and had a fixed gaze. His right pupil was 3 mm and his left pupil 2 mm left, with consensual pupillary reflexes present. His eye fundi were without papilledema, hemorrhages, or exudates. There was evidence of horizontal nystagmus with rapid movement to the right. Other cranial nerves did not show this detected condition. The patient showed the presence of a neck stiffness, with Brudzinski present and a negative Kerning. His tendon reflexes increased in his lower limbs, with bilateral Babinski and a non-assessable walking. The patient demonstrated an asynchronous myoclonus of all extremities that was associated with loud sounds or light, and he had a tendency to turn his head to the right side.
In laboratory tests and during initial and subsequent clinical monitoring [(blood count, blood chemistry, serum electrolytes, liver function tests, gasometry analysis, serological tests for human immunodeficiency virus, hepatitis B, hepatitis C, VDRL, thyroid function tests, chest radiography, abdomen evaluation [
Figure 3
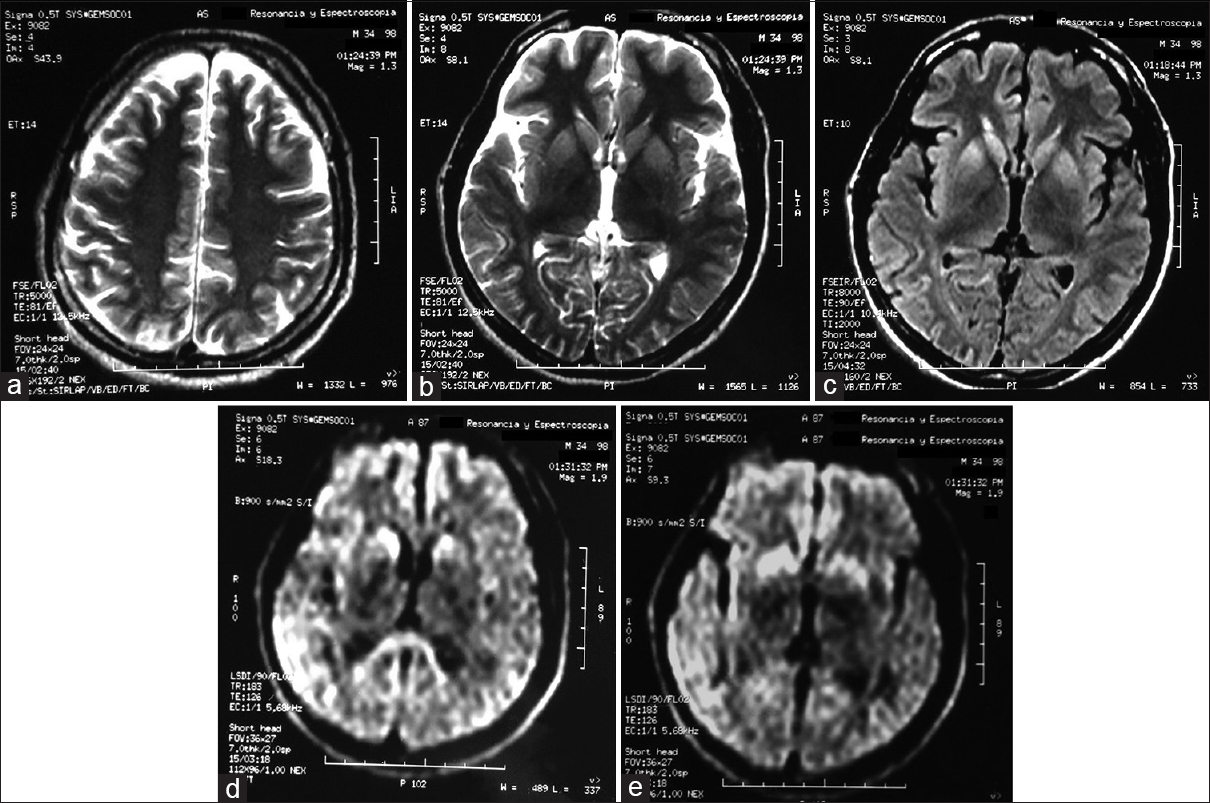
Note the magnetic resonance imaging (MRI) of the skull in the axial T2 sequence where data loss of corticosubcortical volume are observed diffusely (a). MRI in T2 sequence where hyperintensity data are identified at the level of caudate nucleus heads and putamen bilaterally with tendency to symmetry (b). MRI in T2 fluid attenuated inversion recovery (FLAIR) sequence where hyperintensity data are identified at the level of caudate nucleus heads and putamen bilaterally with tendency to symmetry (c). MRI in sequence diffusion (diffusion weighted imaging) where diffusion restriction is identified at the level of caudate nucleus heads, splenius of the corpus callosum (d). MRI in sequence diffusion (diffusion weighted imaging) where diffusion restriction is identified at the level of right temporoparietal cortical region predominantly right cortical region and in both putamen which tends to symmetry (e)
The patient showed a lethargic state of consciousness that was in rapid decline. The patient was handled given his evolution upon admission to the hospital as having a rapidly progressing dementia syndrome. Creutzfeldt–Jakob encephalopathy was ruled out with a brain biopsy performed as a confirmatory diagnosis. The same result was reported with a histopathology, which showed a grayish color occipital tissue with the presence of infectious mycotic cerebral white–gray matter, giving a definitive diagnosis of brain tissue that tested positive for Aspergillus [
Figure 4
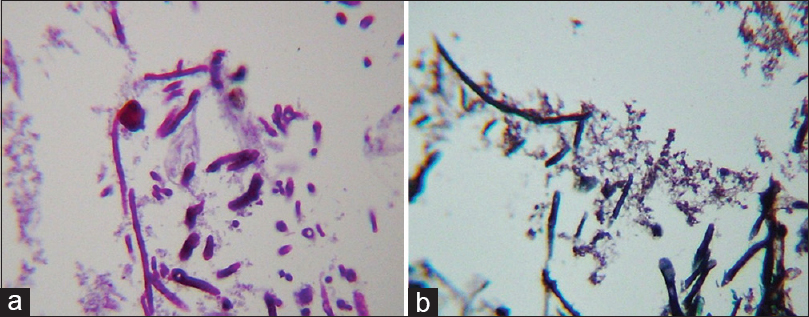
View of hyphae in brain biopsy, showing positivity for periodic acid Schiff (PAS), in which regular hyphae are observed at acute angles, as is a round conidiophore on completion of the hyphae; PAS ×400 (a). Stained hyphae with the Silver technique, showing reinforced walls, regular and septa that were observed with a more reinforced black color, forming acute angles characteristic of Aspergillus walls; Grocottmethenamine silver ×400 (b)
DISCUSSION
According to published literature, the definitive diagnosis of Creutzfeldt–Jakob disease (CJD) is done by biopsy and/or brain autopsy. There are signs and symptoms that guide us. To this end, The World Health Organization (WHO) developed the following diagnostic criteria, listing cases as definitive, probable, and possible:
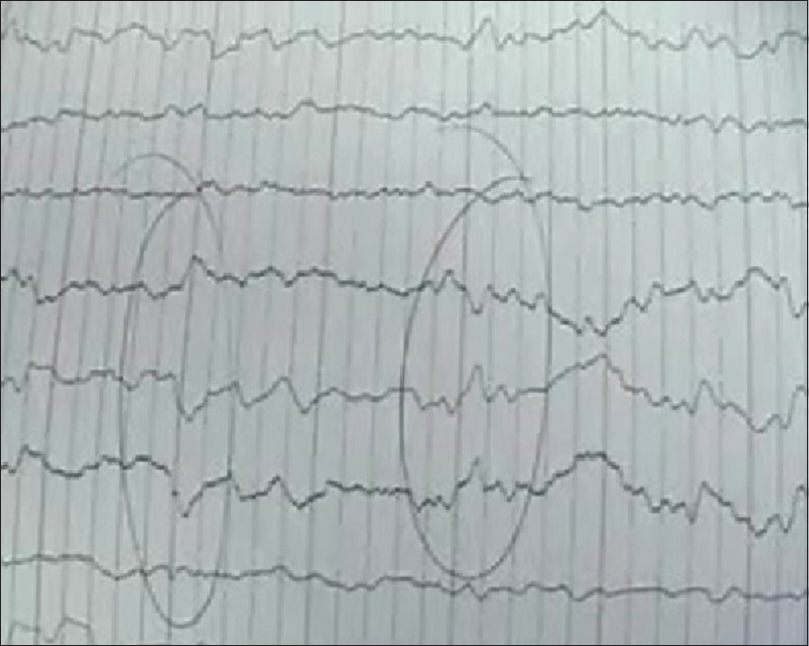
Definitive: Compatible clinical process and detection of prion protein (PrP) in brain tissue, either by Western blot or by histopathology. Probable: Rapidly progressive dementia (<2 years) associated the presence of 14-3-3 protein in CSF and/or changes in typical EEG and having at least two of the following:
- Myoclonus - Ataxia and/or visual signs and symptoms - Akinetic Mutism - Pyramidal or extrapyramidal signs and symptoms Possible: Signs and symptoms of CJD but without EEG nor CSF changes (or that they were not evaluated).[
However, there are 2 other entities to classify the CJS: (1) The iatrogenic syndrome that consisting in a progressive cerebellar syndrome in the patient's hormone pituitary receptor and (2) The family CJS which is diagnosed as a probable or possible CJD along with a close relative with definite CJD.[
Considering the signs and symptoms of the patient and their evolution, laboratory data, where the presence of slightly elevated protein levels in CSF was remarkable according to the age of the patient, along with the erythrocyte count, which suggested the possibility of an intrinsic pathology such as an incipient infectious process. The study of the images pointed toward the differential diagnoses; the most likely diagnoses were of an infectious cerebral process that involved the meninges (considering an altered state of consciousness with episodes progressively alternating between a soporose state and an awakened state in the context of presence of neck stiffness, with a bilateral Babinski, and positive Brudziski) vs. a demented progressive syndrome type of CJD (given the presence of a rapidly progressive dementia, pyramidal signs, akinetic mutism, and mainly myoclonic jerks).
Given the almost specific presentation of CJS, a brain biopsy was performed. The diagnosis was surprising, considering that in medical literature, cases of a cerebral infectious process are very rare in immunocompetent patients. We cannot be sure that the patient was immunocompetent as no complement nor immunoelectrophoresis studies were performed. These studies were not performed as this diagnosis was not suspected given that MRI showed no brain infection process. The clinical and laboratory data were not very specific to contemplate such a diagnosis. The data point toward the CJS and the results of the biopsy were an important finding.
Based on the results obtained by the histopathology of brain biopsy and on the clinical and developmental etiology of our patient, the literature refers to the cerebral aspergillosis as an opportunistic and serious infectious disease that mainly affects immunocompromised patients and which involves 10–20% of the cases of invasive aspergillosis. (These individuals are patients with human immunodeficiency virus (HIV), transplants, a neutropenic disorder, undergoing chemotherapy, taking corticosteroids, or with oncological diseases.) The most common site of invasion is the CNS. This pathology is rare in immunocompetent patients, and focal intracerebral infection is potentially fatal.[
It is common in individuals living in the country and in farmers who are in contact with birds. It is also more prevalent in tropical climates. The most common site of infection in immunocompromised patients is the lungs, although other points of infection are also possible. In immunocompetent patients, the common site of infection is the sinuses.
The most common factors that predispose patients to cerebral aspergillosis are neutropenia and the use of corticosteroids. However, the disease is also prevalent in climates with large numbers of spores, such as in hot, desert, or tropical areas. The following individuals are also potentially predisposed to cerebral aspergillosis: Immunocompromised patients, HIV carriers, organ transplant patients, cancer patients, those on immunosuppressive therapy, individuals with pulmonary arteriovenous malformations, individuals with congenital heart disease, diabetics, patients with maxillofacial infections, those with cytomegalovirus (CMV), patients with chronic granulomatous disease, those with multiple myeloma, sarcoidosis patients, and those with liver failure, penetrating cranioencephalic trauma (CET), or a history of neurosurgery. This disease also affects patients with infections at distant sites with aspergillus in the lungs; to mention a common example, patients with prolonged hospitalizations. Patients at the age of 40 years are also at risk.[
The dissemination routes for the development of cerebral aspergillosis are mainly from the following: Contiguity is the most frequent, being a direct spread of infection. Examples include the otitis media, dental infections, sinusitis, mastoiditis (In this context, although our patient was immunocompetent, it is very possible that the antecedent of head trauma, with broken nose might have led to an asymptomatic sinusitis). Brain abscesses by Aspergillus if developed through this pathway tend to be unique. A second form of spread is by direct inoculation, as in the cases of severe trauma or history of neurosurgery. An additional way is through the hematogenous spread. Here, the spread of infections from distant sites such as the skin, pelvis, lungs, pleura, heart, or intraabdominal comes through the blood to the CNS. It has been reported that this is the route of spread of special predilection by this organism. It has also been shown that this dissemination has a special predilection on the part of the microorganism for the development of abscesses where they appear in both brain hemispheres simultaneously.[
The etiological agents of cerebral aspergillosis are Aspergillus fumigatus, which is the most common in immunocompromised patients and A. flavus, the most common in immunocompetent patients. However, there are more than 200 different types of Aspergillus
that can cause major diseases. Among the species most commonly found are: A. flavus, A. niger, A. terreus, and A. nidulans. A. fumigatus is the most common cause of clinical disorders, and it is a ubiquitous organism found around the world. Other agents of the same species are: A. amstelodami, A. savenaceus, A. candidlus, A. carneus, A. caesiellus, A. clavatus, A. glaucus, A. granulosus, A. oryzae, A. quadrilineatus, A. restrictus, A. sydowi, A. ustus, A. versicolor to name a few.[
The origin of the infectious source of brain abscess is mainly through the upper airway, e.g. the nostrils, mouth, or ear. However, in immunocompetent patients, it is through the sinuses in 60–70% of cases, and in others, a cranial injury is followed by direct inoculation.[
The pathology of aspergillosis in the CNS can be classified in three ways: Infarction, granulomas, and meningitis. The fungal hyphae block intracerebral blood valves resulting in thrombosis and a subsequent heart attack and hemorrhage. Fungi can subsequently spread beyond the valvular walls and form abscesses in the affected brain tissue. Purulent lesions may be chronic and show a tendency toward fibrosis and the formation of granuloma. The erosion of the valve wall may also cause mycotic aneurysms to form.[
However, some medical literature complements and also differs slightly. That is, some literature reports that the invasion of CNS in immunocompetent patients may present itself as multiple alterations of the cranial nerves, cavernous sinus syndrome, orbital sinus invasion syndrome, or a multiple or isolated granuloma, with or without a relationship to meningitis. On the other hand, the CNS invasion in the immunocompromised patient may occur in the multiple areas of cerebral infarction, hemorrhage, encephalomalacia, a cerebrovascular accident (CVA), or as a multiple or isolated abscess.[
The histogenesis of brain abscess occurs in four stages, which are briefly described below:
Stage I: Early cerebritis, wherein perivascular inflammatory infiltration and edema occur; said stage takes from 1 to 3 days Stage II: Late cerebritis, which lasts for 4–9 days and involves the formation of central necrosis Stage III: Early capsule formation, which lasts for 10–14 days and involves necrosis surrounded by swollen infiltrate with macrophages and fibroblasts Stage IV: Vascularized capsule formation, which lasts for more than 14 days and has dense collagen fibers.[
Other presentations of CNS aspergillosis are reported and are uncommon, including basilar meningitis, myelitis, invasion of the carotid artery, dural abscess, and mycotic aneurysm (the latter previously mentioned).[
The most common clinical manifestation of patients with brain abscess is the headache (60% as the most common symptom). The pain is located on the site of the abscess and may be gradual or acute. In addition, there is a concentrated neurological deficit (50%), which occurs from days to weeks after the headache. Other manifestations include: fever (45–50%), papilledema (25%), and seizures (25%). These symptoms may be the first manifestation of a brain abscess, and the most common abscesses are in the frontal lobe.[
To confirm the diagnosis by considering the clinical manifestations presented is difficult because they are not specific; radiological findings can be suggestive, but not pathognomonic.[
With regards to imaging studies, the CTA and MRI (the latter study that provides the most sensitivity are those that provide meaningful data that support the diagnosis.[
In serologic studies, we can mention the antigen detection test for Galactomannan, a heteropolysaccharide found in the cell wall of the Aspergillus. A sample can be obtained from blood, urine, bronchoalveolar lavage or CSF.[
Another study to consider for diagnosis is the test of Beta-D circulating glucan, a polysaccharide component of the cell wall of most fungi species except those of the of Zycomycetes cryptococci. Test results have found that sensitivity is 55–100% and specificity is 78–100%. For the diagnosis, monitoring, and treatment of patients with invasive aspergillosis in neutropenic adults, one can also compare the usefulness and purpose of aforementioned study against the Galactomannan antigen determination of ELISA. It is reported that the combination of both tests increases both sensitivity and specificity.[
A positive culture of a sample taken directly from a usually sterile site (for example, a brain abscess), or Positive results of both the histology and culture sample taken from an affected organ.[
With respect to the treatment of cerebral aspergillosis, there are some treatments that have proven effective. Amphotericin B deoxycholate has been a first-rate antifungal therapy at doses of 0.8–1.25 mg/kg/day. It is taken intravenously according to the patient response and serum creatinine levels.[
It is necessary to note that in some patients amphotericin B deoxycholate has been ineffective. The inadequacy of this drug is likely because of the very advanced stage of the disease and a late diagnosis. To solve this problem, some future therapeutic options have been raised in order to prevent antifungal resistance by using granulocyte colony stimulating factor (G-CSF) and interferon gamma.[
Regarding a new therapeutic treatment cerebral aspergillosis, the use of Caspofungin has been proposed. It has been approved with initial doses of 70 mg/day, followed by 50 mg/day, both intravenous given that there is no oral option.[
In longitudinal studies, Voriconazole versus Amphotericin has shown a more effective response to 12 weeks (53% versus 32%, respectively), and the reduction in mortality has been 29% against 42%, respectively, for patients with invasive aspergillosis. The doses used in this study were for Voriconazole 6 mg/kg intravenously every 12 h for 1 day, and then 4 mg/kg intravenously every 12 h for 7 days. For amphotericin, the doses were 1 to 1.5 mg/kg per day.[
The prognosis for patients with invasive aspergillosis SNC is very bad in the short term, with an overall mortality rate of over 90% with treatment, mortality reaching 100% of cases without treatment. However, with proper and timely treatment, the possibilities of a complete cure can reach up to 35%.[
CONCLUSION
Cerebral aspergillosis is an aggressive disease, rare in patients with respected immunity. The disease is common in immunosuppressed patients with nonspecific clinical manifestations. These manifestations are mostly characterized by an alteration of consciousness, seizures, headaches, and focal neurological signs. An accurate diagnosis is made by brain biopsy, with very poor prognosis in the short term and an encouraging short-term efficacy of antifungal medicine administered in a timely manner.
The suspected diagnosis should also be considered in any patient whose impairment of higher mental functions is progressing or evolving rapidly. This diagnosis is above all the case if there is a history of craniofacial trauma in the short and medium term (pending objectively establishing the time elapsed since said medical history). Early diagnosis is the only opportunity to improve the chances of an aggressive response to treatment given in a timely manner. With proper follow-up, the prognosis of these patients would improve.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Aliff TB, Maslak PG, Jurcic JG, Heaney ML, Cathcart KN, Sepkowitz KA. Refractory Aspergillus pneumonia in patients with acute leukemia: Successful therapy with combination caspofungin and liposomal amphotericin. Cancer. 2003. 97: 1025-32
2. Arango-Lasprilla JC, Fernández-Guinea S, Ardila A.editorsDementias: clinical, neuropsychological aspects and treatment. Single Volume. Mexico: Manual Moderno; 2003. p. 17-
3. Chun CH, Johnson JD, Hofstetter M, Raff MJ. Brain abscess. A study of 45 consecutive cases. Medicine (Baltimore). 1986. 65: 415-31
4. Del Bono V, Mikulska M, Viscoli C. Invasive aspergillosis: Diagnosis, prophylaxis and treatment. Curr Opin Hematol. 2008. 15: 586-93
5. Denning DW, Ribaud P, Milpied N, Caillot D, Herbrecht R, Thiel E. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin Infect Dis. 2002. 34: 563-71
6. DíazMartínez JC, Takeuchi-Tan Y. Creutzfeldt-Jakob disease: Clinical, electroencephalografic, pathologic, and imaging characteristics. Acta Neurol Colomb. 2008. 24: 118-23
7. Erdogan E, Beyzadeoglu M, Arpaci F, Celasun B. Cerebellar Aspergillosis: Case report and literature review. Neurosurgery. 2002. 50: 874-6
8. Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL.editorsHarrison's Principles of Internal Medicine: Aspergillosis. Single Volume. New York: McGraw-Hill Medical; 2008. p. 1259-60
9. Friedlander RM, Gonzalez RG, Afridi NA, Pfannl R. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 16-2003. A 58-year-old woman with left-sided weakness and a right frontal brain mass. N Engl J Med. 2003. 348: 2125-32
10. Garcia RJ, Troya P, Edwards C. Invasive Aspergillosis with central nervous system dissemination in a presumably immunocompetent, non neutropenic patient: Case report and review. South Med J. 2006. 99: 607-10
11. Gueret R, Patel GP, Simon D, Balk RA. Invasive Aspergillosis Case report and review of the approach to diagnosis and treatment. Clin Pulmonary Med. 2007. 14: 197-205
12. Hao L, Jing C, Bowen C, Min H, Chao Y. Aspergillussellar abscess: Case report and review of the literature. Neurol India. 2008. 56: 186-8
13. Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002. 347: 408-15
14. Jain KK, Mittal SK, Kumar S, Gupta RK. Imaging features of central nervous system fungal infections. Neurol India. 2007. 55: 241-50
15. Klont RR, Meis JF, Verweij PE. Critical assessment of issues in the diagnosis of invasive aspergillosis. Clin Microbiol Infect. 2001. 7: 32-7
16. Kowacs PA, Monteiro de Almeida S, Pinheiro RL, Fameli H, Piovesan EJ, Correia A, Werneck LC. Central nervous system Aspergillus fumigatus infection after near drowning. J Clin Pathol. 2004. 57: 202-4
17. Maertens J, Van Eldere J, Verhaegen J, Verbeken E, Verschakelen J, Boogaerts M. Use of circulating galactomannan screening for early diagnosis of invasive aspergillosis in allogeneic stem cell transplant recipients. J Infect Dis. 2002. 186: 1297-306
18. Mathisen GE, Johnson JP. Brain abscess. Clin Infect Dis. 1997. 25: 763-79
19. Saballs P, López Colomés JL, Cobos JG, Knobel H. Invasive aspergillosis: treatment. Ibero-American Journal of Mycology. 2000. 17: S93-6
20. Scully EP, Baden LR, Katz JT. Fungal brain infections. Curr Opin Neurol. 2008. 21: 347-52
21. Segal BH. Aspergillosis. N Engl J Med. 2009. 360: 1870-84
22. Seydoux C, Francioli P. Bacterial brain abscesses: Factors influencing mortality and sequelae. Clin Infect Dis. 1992. 15: 394-401
23. Stevens DA, Kan VL, Judson MA, Morrison VA, Dummer S, Denning DW. Practice guidelines for diseases caused by Aspergillus. Infectious Diseases Society of America. Clin Infect Dis. 2000. 30: 696-709
24. Tattevin P, Bruneel F, Clair B, Lellouche F, de Broucker T, Chevret S. Bacterial brain abscess: A retrospective study of 94 patients admitted to an intensive care unit (1980 to 1999). Am J Med. 2003. 115: 143-6
25. Walsh TJ, Shoham S, Petraitiene R, Sein T, Schaufele R, Kelaher A. Detection of galactomannan antigenemia in patients receiving piperacillin-tazobactam and correlations between in vitro, in vivo, and clinical properties of the drug-antigen interaction. J Clin Microbiol. 2004. 42: 4744-8