- Department of Neurosurgery, Sapienza University of Rome, Rome, Italy
- Department of Neurosurgery, “Sant’Eugenio” Hospital, Rome, Italy.
Correspondence Address:
Anthony Kevin Scafa, Department of Neurosurgery, Sapienza University of Rome, Rome, Italy.
DOI:10.25259/SNI_1068_2022
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Anthony Kevin Scafa1, Tingting Jiang1, Lorenzo Pescatori2, Massimo Corsini1, Manolo Piccirilli1. Association between spontaneous intracranial epidural hematoma and craniofacial infections: A systematic literature review. 17-Feb-2023;14:57
How to cite this URL: Anthony Kevin Scafa1, Tingting Jiang1, Lorenzo Pescatori2, Massimo Corsini1, Manolo Piccirilli1. Association between spontaneous intracranial epidural hematoma and craniofacial infections: A systematic literature review. 17-Feb-2023;14:57. Available from: https://surgicalneurologyint.com/surgicalint-articles/12158/
Abstract
Background: Spontaneous and nontraumatic epidural hematoma (SEDH) is a rare entity. Etiology is various, including vascular malformations of the dura mater, hemorrhagic tumors, and coagulation defects. The association between SEDH and craniofacial infections is rather unusual.
Methods: We performed a systematic review of the available literature using the PubMed, Cochrane Library, and Scopus research databases. Literature research was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. We exclusively included studies reporting demographic and clinical data, published until October 31, 2022. We also report one case from our experience.
Results: A total of 18 scientific publications, corresponding to 19 patients, met the inclusion criteria for the qualitative and quantitative analysis. Patients were mostly adolescents, with a clear male predominance. SEDHs frequently occurred in the frontal area, usually near the site of the infection. Surgical evacuation was the treatment of choice with good postoperative outcomes. Endoscopy of the involved paranasal sinus should be achieved as soon as possible to remove the cause of the SEDH.
Conclusion: SEDH may occur as a rare and life-threatening complication of craniofacial infections; therefore, prompt recognition and treatment are mandatory.
Keywords: Epidural, Hematoma, Infection, Intracranial, Spontaneous, Surgery and antibiotics
INTRODUCTION
An epidural hematoma (EDH) is an extra-axial collection of blood lying between the outer layer of the dura mater and the inner table of the skull. It is generally associated with head trauma, occurring in about 2% of all head injuries with mortality rates ranging from 1.2% to 33%.[
Spontaneous EDH (SEDH) is a rare entity. Even though it has been described as “spontaneous,” an underlying cause has been recognized in all the reported cases. Etiology includes vascular malformations of the dura mater, hemorrhagic tumors, coagulation defects, and but also craniofacial infections.[
To date, only 19 cases of SEDH associated with craniofacial infections have been reported in the literature.[
We discuss the relevant literature and report one case from our experience.
MATERIALS AND METHODS
Study design
The present investigation consists of a case report and a systematic review of the literature conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.
Eligibility criteria
All written papers about the association between spontaneous intracranial EDH and craniofacial infections, reporting demographic and clinical data, diagnostic workflow, treatment, and prognosis were considered for eligibility. Articles lacking precise information were excluded, together with surgical and radiological technical notes, anatomical studies, abstracts from scientific meetings, and unpublished reports; articles discussing nonspontaneous EDHs were considered “not eligible” as well.
Publications written in languages other than English were considered in our paper only if the relevant information could be inferred from a complete abstract written in English.
Information sources and search strategy
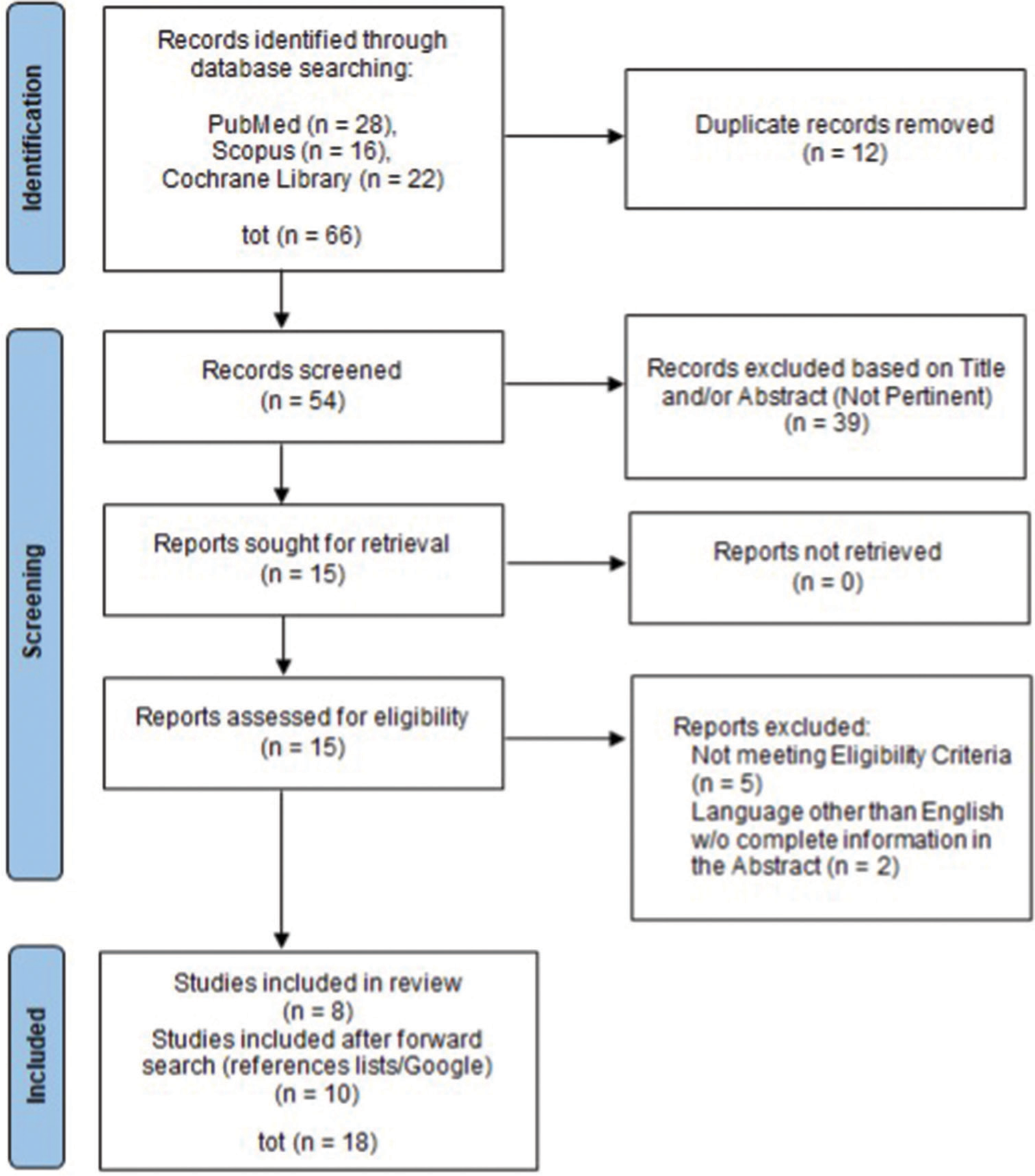
The systematic review of the literature was performed on three different online medical databases (PubMed, Cochrane Library, and Scopus), using as search-terms “spontaneous,” “epidural,” “extradural,” “hematoma,” and “sinusitis” (being otitis and mastoiditis considered as falling under this definition) (All Fields), combined with the Boolean operators “OR” and “AND.” The last search was conducted on October 31, 2022, and went back as far as data were available. The search strategy is summarized in
Data collection process and study selection
Abstracts and full texts were independently screened by two authors (Anthony Kevin Scafa and Massimo Corsini), and any discordance was solved by consensus with a third, senior author (Manolo Piccirilli). Reference lists of the included full texts (“forward search”) were similarly screened and evaluated for inclusion.
RESULTS
After duplication removal, 54 papers were screened for this systematic review. Thirty-nine papers were excluded because “not pertinent” (exclusion criterion), and 15 full-text papers were, therefore, evaluated. Among these, seven papers were excluded according to our eligibility criteria so that eight papers, plus 10 from forward search, were finally included and analyzed[
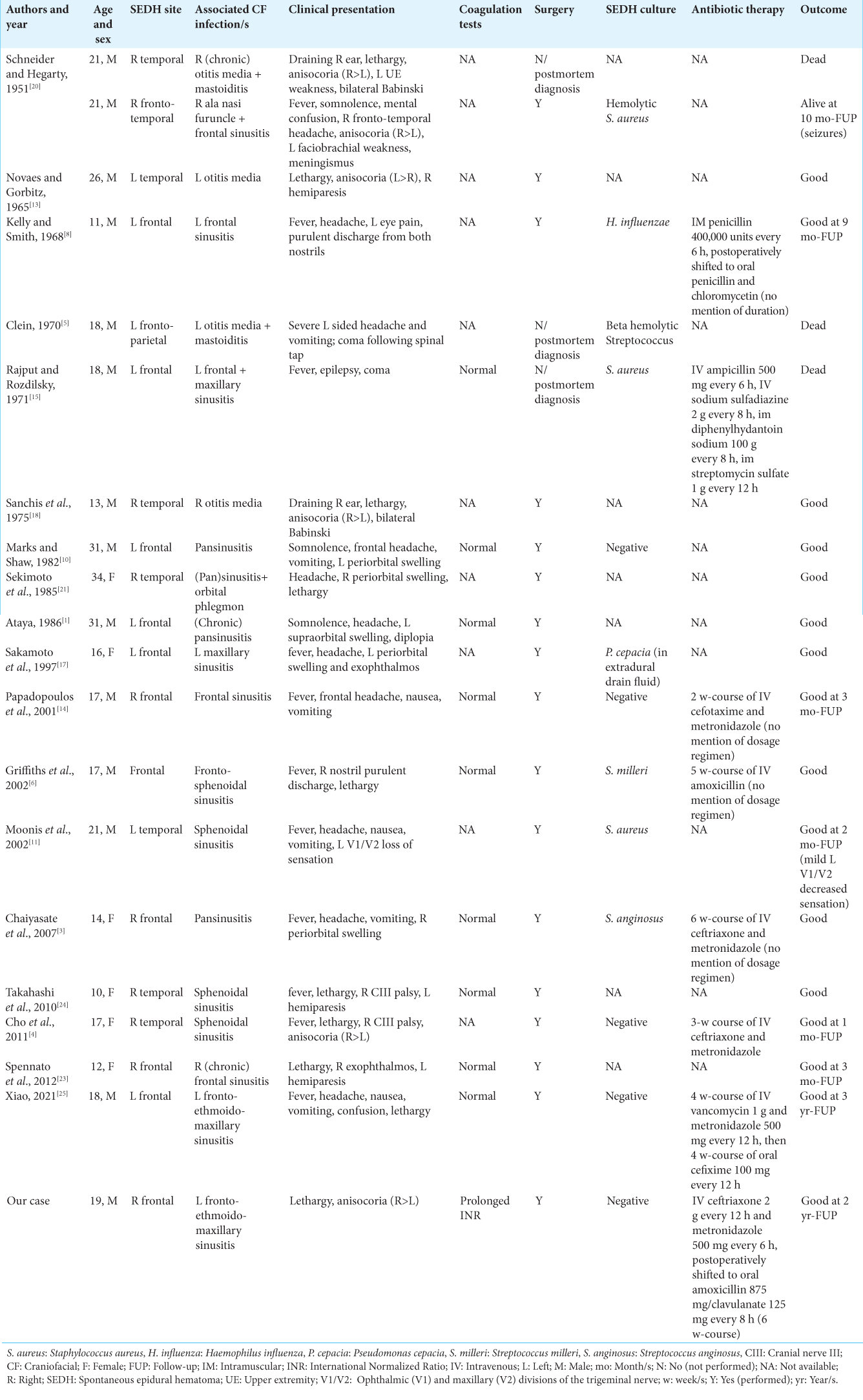
The mean age of the investigated patients (n = 20, 19 from the literature review and 1 from our experience) was 19.3 (range 10–34 years). The M: F ratio was 2.3:1.
Setting of the hemorrhage was supratentorial in all cases; location was frontal (alone or in combination with adjacent sites) in 13 patients (65%).
The “cause” of the hemorrhage was an infection of the frontal sinus (alone or in combination with infection of the adjacent sinuses) in 12 cases (60%).
Clinical profiles were diverse and are reported in
Coagulation tests were normal in all the cases for which this information was available, apart from our case.
SEDH was diagnosed postmortem in three cases (15%); surgery was the treatment of choice in the remaining 17 patients (85%), with good postoperative outcomes in all cases.
Eight patients (40%) had positive intraoperative cultures (Gram-positive cocci in six cases, 75%). Results were negative in 5 (25%), not available in 7 (35%) [
Information regarding antibiotic therapy was available in only seven cases [
CASE REPORT
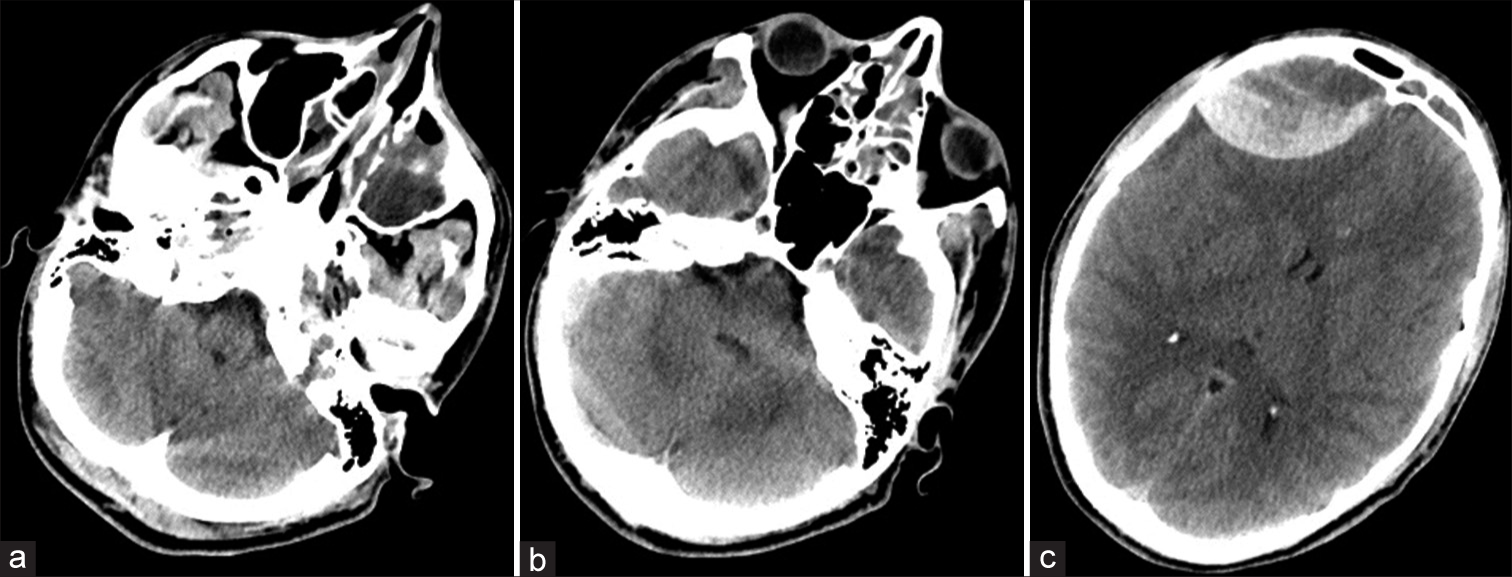
A 19-year-old male was admitted to the emergency department of a peripheral hospital complaining of headache, agitation, vomiting, diplopia, and fever. The patient suffered from left conjunctivitis, treated with antibiotic eyewashes, 3 weeks earlier, and had a history of headache in the past 10 days. His parents denied any history of trauma or intake of any kind of drug, except for nonsteroidal anti-inflammatory drugs (NSAIDs) (Ketoprofen 80 mg, twice a day, for 10 days). On neurological examination, the patient opened his eyes with verbal and painful stimuli; neither focal deficits nor nuchal rigidity was detected (GCS = 14). The CT scan of the brain (plain and bone windows) revealed a large hyperdense biconvex-shaped mass in the right frontal region (6.5 × 3.5 cm) which compressed the surrounding cerebral parenchyma, without evidence of skull fractures. The CT scan did not show signs of pneumocephalus. Furthermore, inflammatory tissue was highlighted in the left ethmoidal cells and in the frontal and maxillary sinuses [
Few hours later, the patient became drowsier and confused and developed right anisocoria (right: left pupillary diameter = 4:2). A new CT scan of the brain was immediately obtained, and an increase in the volume of the right frontal hyperdense mass was noticed (8 × 4.7 cm). The patient was intubated and immediately transferred to our neurosurgical operating theater.
A right frontal craniotomy was performed, and a voluminous EDH was evacuated. Intraoperatively, no skull fracture was detected. Similarly, neither dural nor bony vascular malformation was appreciated. The source of bleeding was found in a branch of the right MMA. Histological examination of the intraoperative material pointed out fibrin and leukocytes compatible with hematoma.
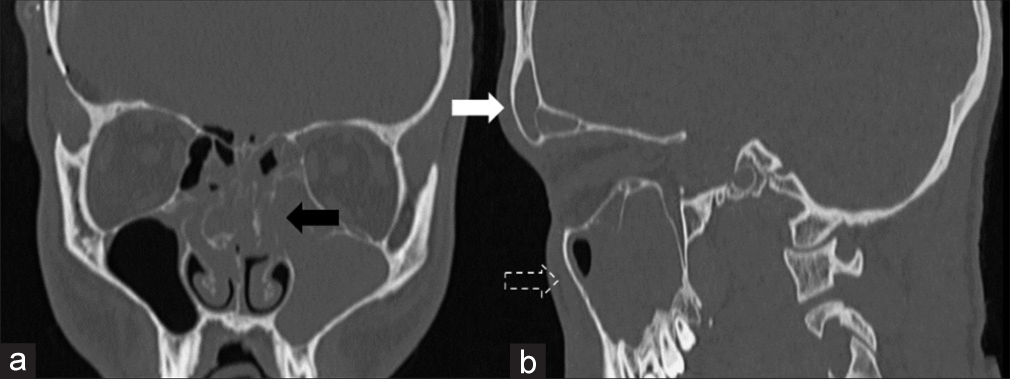
After surgery, the patient’s anisocoria gradually decreased until he regained isocoria. The postoperative brain CT scan showed a good radiological outcome. The patient was, then, transferred to the Neurosurgical Intensive Care Unit and was started on a regimen of intravenous ceftriaxone 2 g twice a day and metronidazole 500 mg 4 times a day, as advised by the infectious disease specialist. He was extubated on postoperative day 2. The INR value spontaneously normalized 2 days later. Two weeks after the neurosurgical procedure, a high-resolution CT scan with the study of paranasal sinuses was performed [
Postoperative laboratory tests were negative for immunologic diseases and coagulopathies.
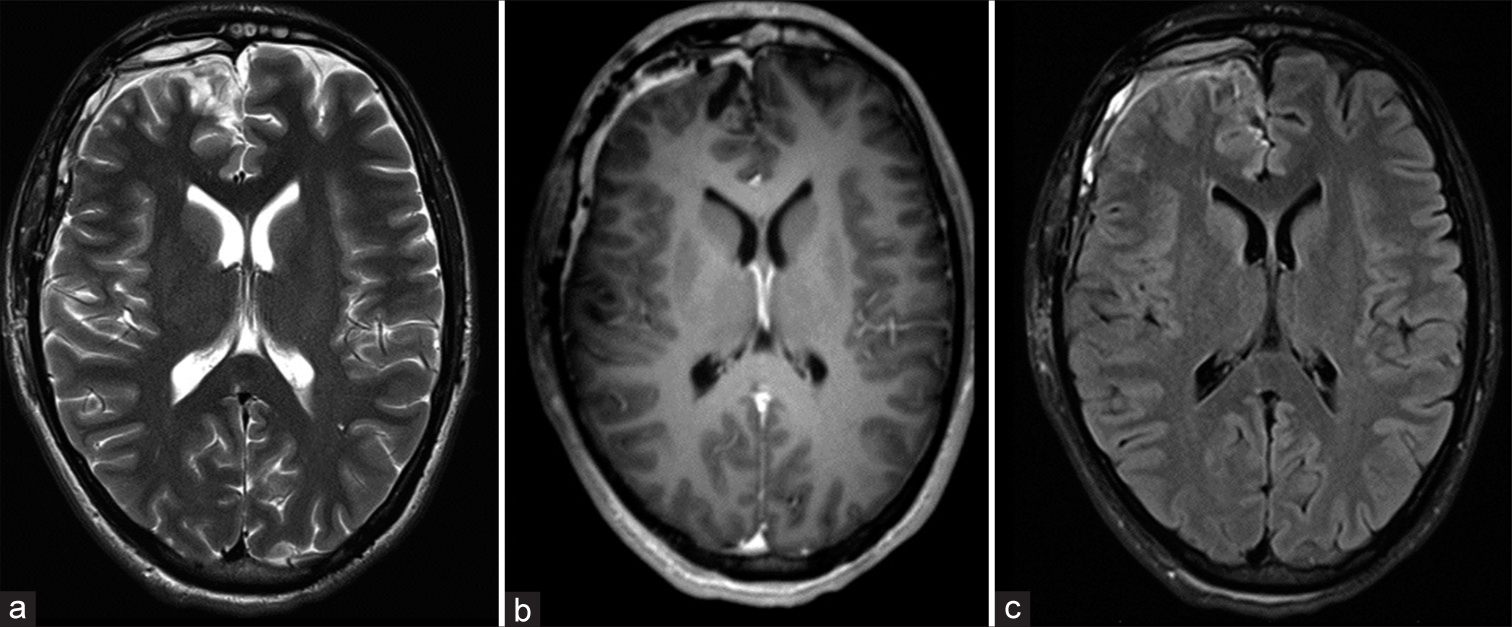
The patient fully recovered without neurological deficits and was discharged home on oral antibiotic therapy for a total of 6 weeks. A brain magnetic resonance imaging performed 1 month after neurosurgical treatment on suggestion of the infectious disease specialist showed a good radiological outcome [
DISCUSSION
Craniofacial infections can lead to severe cerebral complications, such as extra- or intradural empyema, meningitis, cerebral abscess, and venous thrombosis. In some rare cases, these infections are associated with SEDHs.[
The first two cases of SEDH associated with craniofacial infection were described by Schneider and Hegarty[
As previously stated, SEDH is a rare entity and only nineteen cases associated with craniofacial infection have been described so far[
Patients are generally adolescents, and a clear male predominance has been demonstrated. SEDHs frequently occur in the frontal area, usually near the site of infection. The most common neurological reported “symptoms” are headache, confusion, diplopia, and drowsiness; general “symptoms” such as fever, nausea, and vomiting are reported as well.
None of the previously analyzed cases was related to a history of head trauma. Moreover, none of the patients enrolled had evidence of an increased bleeding risk condition or of a vascular malformation.
Two pathogenetic hypotheses can explain the origin of the SEDH in these patients.
First, craniofacial inflammatory conditions – like frontal sinus infections – may spread through diploic vascular channels and weaken meningeal vessels, causing arteritis. Damaged vessels are prone to bleed, even after mild trauma such as “barotrauma” (coughing, sneezing, or similar). The histological examination can confirm this theory, showing the presence of polymorphonuclear cells infiltration of intracranial tissues.[
The first theory is probably the most appropriate for our case, given the absence of pneumocephalus and of any interruption in the bony wall of the inflamed sinus. Yet, it is also true, as reported in the literature, that, in most cases, potential bone defects cannot be macroscopically identified.[
We believe that the use of nonsteroidal anti-inflammatory drugs (NSAIDs) plays a key role in both these circumstances. However, NSAIDs reduce platelet aggregation by inhibition of the enzyme cyclooxygenase and the production thromboxane A2.[
Although rare, SEDHs should be promptly recognized and surgically treated to avoid permanent neurological deficits and fatal consequences. Laboratory and imaging examinations are important to rule out other causes of SEDH. In the case of a patient with sinusitis and acute onset of headache, nausea and vomiting, neurological deficits, or drowsiness, a brain CT scan should be immediately carried out to exclude a SEDH. If an EDH is diagnosed, immediate neurosurgical treatment coupled with antibiotic therapy can lead to a full patient’s recovery.
At present, no guidelines exist for the antibiotic treatment of this condition, due to the scarcity of cases within a consistent lapse of time. However, it is still advisable to give antibiotics even in case of negative cultures since, as pointed out by Xiao,[
To conclude, endoscopic treatment of the involved paranasal sinus should be achieved as soon as possible to remove the supposed primary cause of the EDH.[
CONCLUSION
Spontaneous and nontraumatic EDH is a rare entity. The association between this problem and craniofacial infections is rather unusual. Prompt recognition and treatment are mandatory to avoid permanent neurological morbidity and mortality.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Ataya NL. Extradural haematoma secondary to chronic sinusitis: A case report. J Laryngol Otol. 1986. 100: 951-3
2. Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW. Surgical management of acute epidural hematomas. Neurosurgery. 2006. 58: S7-15 discussion Si-iv
3. Chaiyasate S, Halewyck S, Van Rompaey K, Clement P. Spontaneous extradural hematoma as a presentation of sinusitis: Case report and literature review. Int J Pediatr Otorhinolaryngol. 2007. 71: 827-30
4. Cho KS, Cho WH, Kim HJ, Roh HJ. Epidural hematoma accompanied by oculomotor nerve palsy due to sphenoid sinusitis. Am J Otolaryngol. 2011. 32: 355-7
5. Clein LJ. Extradural hematoma associated with middle-ear infection. Can Med Assoc J. 1970. 102: 1183-4
6. Griffiths SJ, Jatavallabhula NS, Mitchell RD. Spontaneous extradural haematoma associated with craniofacial infections: Case report and review of the literature. Br J Neurosurg. 2002. 16: 188-91
7. Hassan MF, Dhamija B, Palmer JD, Hilton D, Adams W. Spontaneous cranial extradural hematoma: Case report and review of literature. Neuropathology. 2009. 29: 480-4
8. Kelly DL, Smith JM. Epidural hematoma secondary to frontal sinusitis. Case report. J Neurosurg. 1968. 28: 67-9
9. Khairat A, Waseem M, editors. Epidural hematoma. StatPearls. Treasure Island, FL: StatPearls Publishing; 2022. p.
10. Marks SM, Shaw MD. Spontaneous intracranial extradural hematoma. Case report. J Neurosurg. 1982. 57: 708-9
11. Moonis G, Granados A, Simon SL. Epidural hematoma as a complication of sphenoid sinusitis and epidural abscess: A case report and literature review. Clin Imaging. 2002. 26: 382-5
12. Ng WH, Yeo TT, Seow WT. Non-traumatic spontaneous acute epidural haematoma--report of two cases and review of the literature. J Clin Neurosci. 2004. 11: 791-3
13. Novaes V, Gorbitz C. Extradural hematoma complicating middle-ear infection. Report of a case. J Neurosurg. 1965. 23: 352-3
14. Papadopoulos MC, Dyer A, Hardwidge C. Spontaneous extradural haematoma with sinusitis. J R Soc Med. 2001. 94: 588-9
15. Rajput AJ, Rozdilsky B. Extradural hematoma following frontal sinusitis. Report of a case and review of the literature. Arch Otolaryngol. 1971. 94: 83-6
16. Rincon S, Gupta R, Ptak T. Imaging of head trauma. Handb Clin Neurol. 2016. 135: 447-7
17. Sakamoto T, Harimoto K, Inoue S, Konishi A. Extradural hematoma following maxillary sinusitis. Case illustration. J Neurosurg. 1997. 87: 132
18. Sanchis JF, Orozco M, Cabanes J. Spontaneous extradural haematomas. J Neurol Neurosurg Psychiatry. 1975. 38: 577-80
19. Schafer AI. Effects of nonsteroidal anti-inflammatory therapy on platelets. Am J Med. 1999. 106: 25S-36
20. Schneider RC, Hegarty WM. Extradural hemorrhage as a complication of otological and rhinological infections. Ann Otol Rhinol Laryngol. 1951. 60: 197-206
21. Sekimoto T, Nakagawa Y, Ueda S, Hirakawa K, Fukuma S, Taketomo S. Spontaneous epidural hematoma-report of two cases. No Shinkei Geka. 1985. 13: 1253-7
22. Soon WC, Marcus H, Wilson M. Traumatic acute extradural haematoma-indications for surgery revisited. Br J Neurosurg. 2016. 30: 233-4
23. Spennato P, De Paulis D, Bocchetti A, Pipola AM, Sica G, Galzio RJ. Spontaneous intracranial extradural haematoma associated with frontal sinusitis and orbital involvement. Neurol Sci. 2012. 33: 435-9
24. Takahashi Y, Hashimoto N, Hino A. Spontaneous epidural hematoma secondary to sphenoid sinusitis-case report. Neurol Med Chir (Tokyo). 2010. 50: 399-401
25. Xiao Z. A case report of frontal spontaneous epidural hematoma associated with cranial osteomyelitis and epidural abscess due to paranasal sinusitis. Surg Neurol Int. 2021. 12: 478