- Department of Neurological Surgery, Yoshida Hospital, Cerebrovascular Institute, Kobe, Japan.
Correspondence Address:
Atsushi Matsumoto, Department of Neurological Surgery, Yoshida Hospital, Cerebrovascular Institute, Kobe, Japan.
DOI:10.25259/SNI_244_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Atsushi Matsumoto, Hiroaki Hanayama, Hiroaki Matsumoto, Yusuke Tomogane, Hiroaki Minami, Atsushi Masuda, Ikuya Yamaura, Yutaka Hirata, Yasuhisa Yoshida. Asymmetric posterior reversible encephalopathy syndrome secondary to epilepsy occurring in the chronic phase of subarachnoid hemorrhage. 08-Apr-2022;13:129
How to cite this URL: Atsushi Matsumoto, Hiroaki Hanayama, Hiroaki Matsumoto, Yusuke Tomogane, Hiroaki Minami, Atsushi Masuda, Ikuya Yamaura, Yutaka Hirata, Yasuhisa Yoshida. Asymmetric posterior reversible encephalopathy syndrome secondary to epilepsy occurring in the chronic phase of subarachnoid hemorrhage. 08-Apr-2022;13:129. Available from: https://surgicalneurologyint.com/surgicalint-articles/11521/
Abstract
Background: Posterior reversible encephalopathy syndrome (PRES) is a rare clinical syndrome that refers to a disorder with reversible subcortical vasogenic brain edema involving the parieto-occipital lobe, temporal lobe, basal ganglia, and its surroundings. Radiologically, it is characterized by symmetrical lesions; however, atypical findings have sometimes been reported.
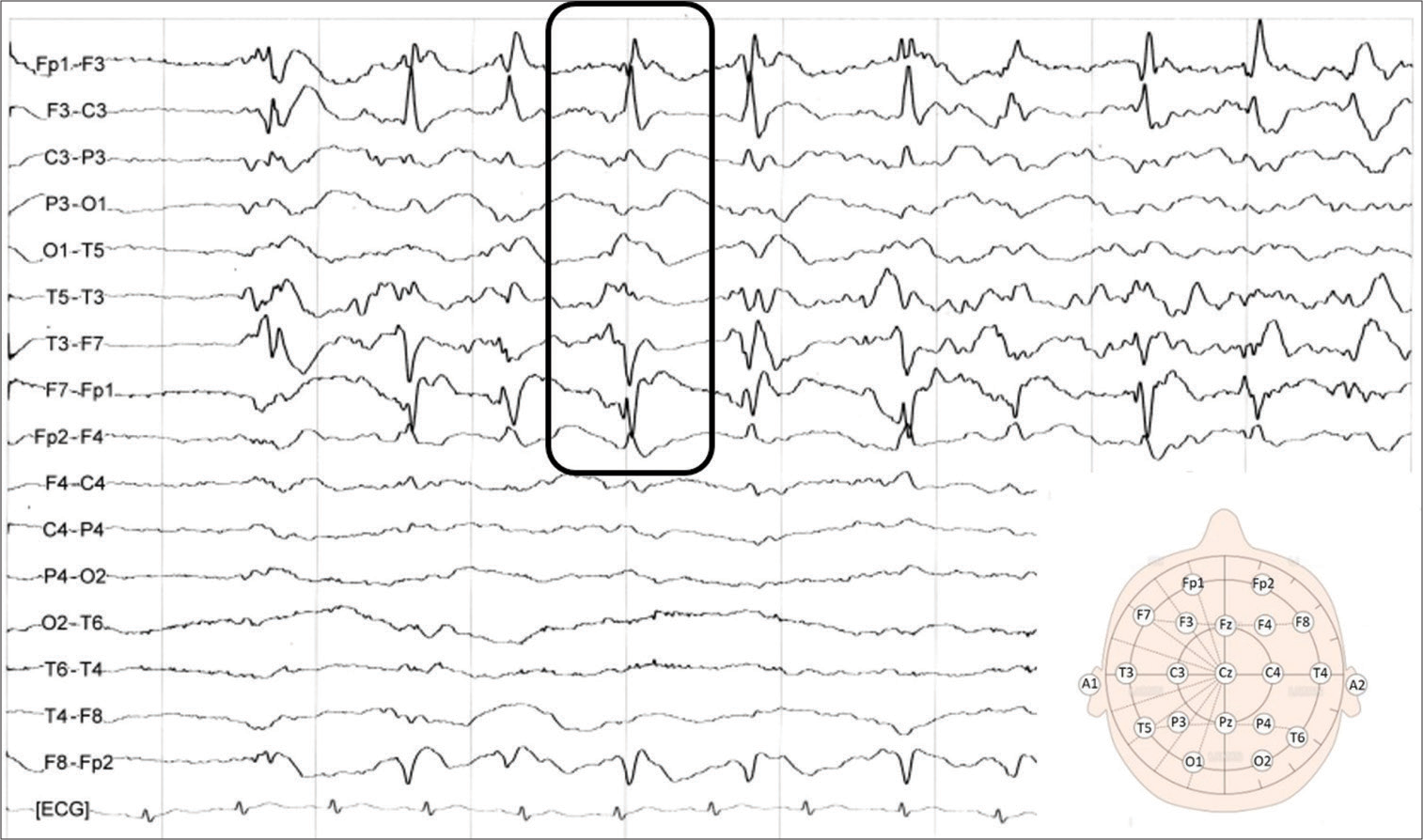
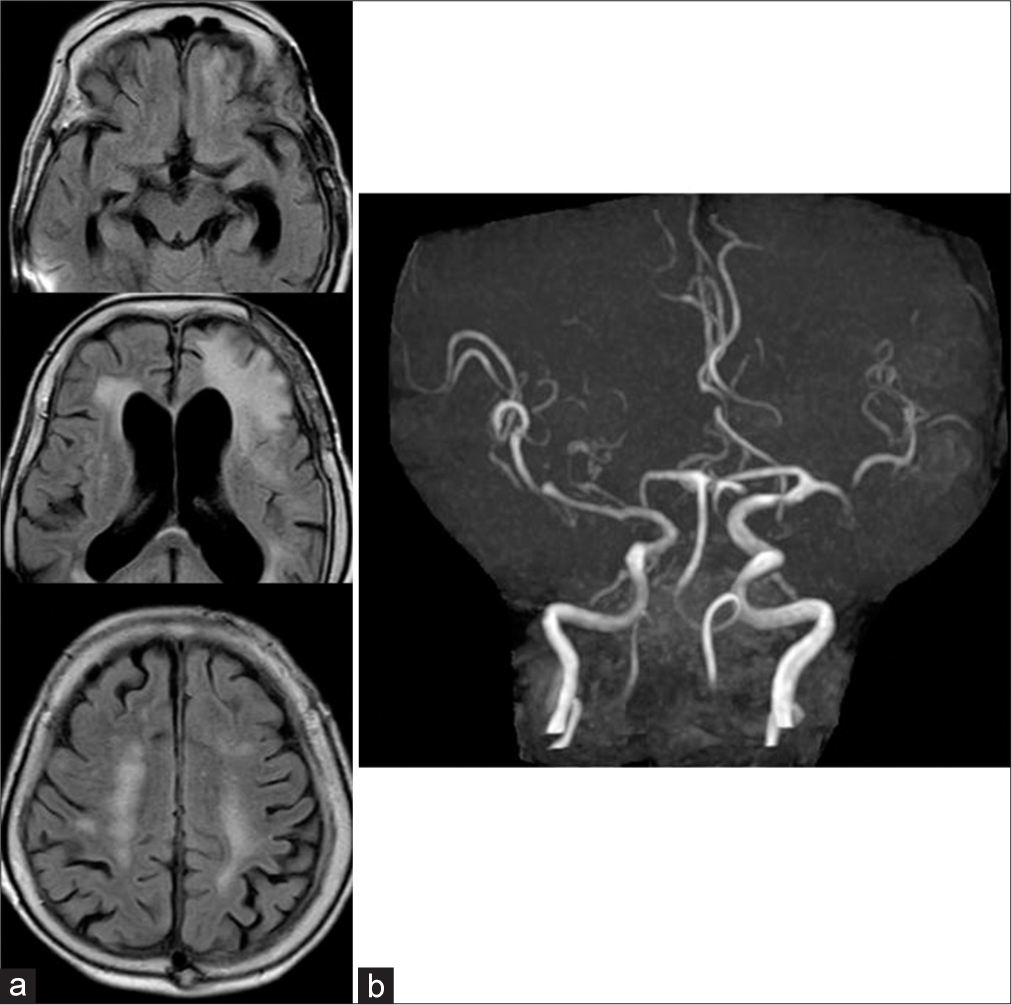
Case Description: A 79-year-old woman experienced subarachnoid hemorrhage (SAH) a year and a half previously before this hospitalization. She presented with sudden-onset coma, dacryorrhea, and moderate right hemiparesis and was taken to our hospital. Computed tomography showed no apparent abnormal acute lesions. Electroencephalography confirmed periodic lateralized epileptiform discharges in the left hemisphere. First, based on the findings, she was diagnosed with nonconvulsive status epilepticus and started antiepileptic therapy. Six days after admission, however, multiple asymmetric lesions were confirmed on magnetic resonance imaging. Considering that findings subsequently improved, we finally diagnosed her with asymmetric PRES secondary to epilepsy occurring in the chronic phase of SAH. Aphasia and right hemispatial neglect persisted as sequelae and she was transferred to a rehabilitation hospital with a modified Rankin scale of 3.
Conclusion: Excessive elevation of blood flow in the hemisphere is inferred to lead to blood–brain barrier collapse and subsequent asymmetric PRES.
Keywords: Asymmetric posterior reversible encephalopathy syndrome, Blood–brain barrier, Epilepsy, Subarachnoid hemorrhage, Vasogenic brain edema
INTRODUCTION
Posterior reversible encephalopathy syndrome (PRES) is a rare clinical syndrome that refers to a disorder with reversible subcortical vasogenic brain edema involving the parieto-occipital lobe, temporal lobe, basal ganglia, and its surroundings. Patients diagnosed with PRES may require intensive care monitoring and treatment due to severe complications, such as epilepsy attack, cerebral ischemia, intracranial hemorrhage, or increased intracranial pressure. Few specific affections such as hypertensive encephalopathy, eclampsia, bacterial infections, transplantation, renal failure, autoimmune disorders, and some kinds of drugs are considered to induce this condition. Radiologically, it is characterized by symmetrical lesions; however, atypical findings have sometimes been reported. We herein report a case of asymmetric PRES secondary to epilepsy occurring in the chronic phase of subarachnoid hemorrhage (SAH). In addition, we summarize and review this subject, as this condition is extremely rare.
CASE DESCRIPTION
A 79-year-old woman, who had experienced SAH of a Hunt and Kosnik Grade II a year and a half previous, presented with sudden-onset coma, dacryorrhea, and moderate right hemiparesis and was taken to our hospital. She had undergone aneurysmal neck clipping for a ruptured internal carotid artery-posterior communicating artery aneurysm and ventriculoperitoneal (VP) shunt placement during her first hospitalization. She was discharged with 1 of modified Rankin scale (mRS) score and had a good clinical course.
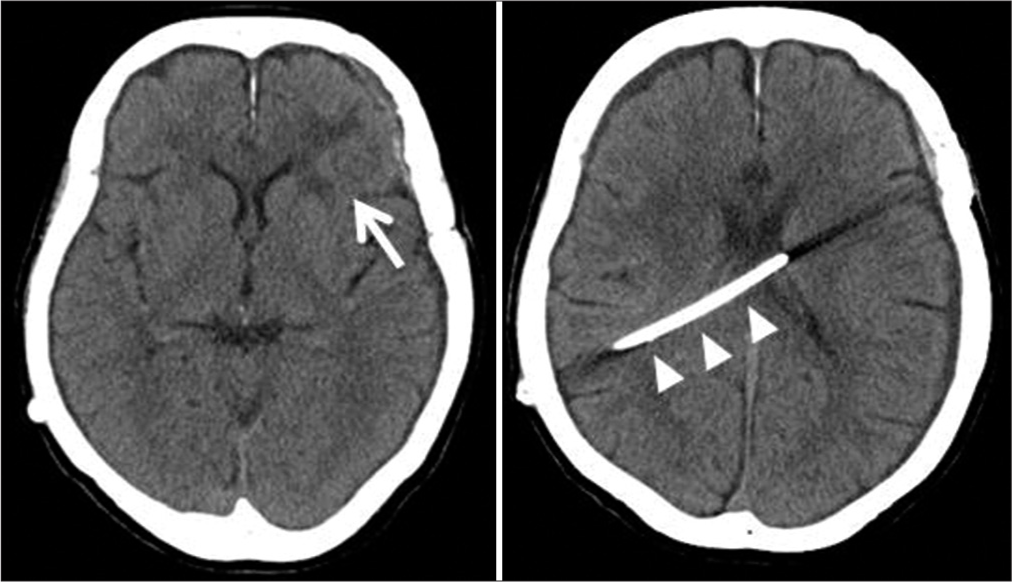
On admission, computed tomography showed no apparent abnormal lesions, except for the left frontal cerebral lobe contusion that occurred in the SAH treatment [
Figure 3:
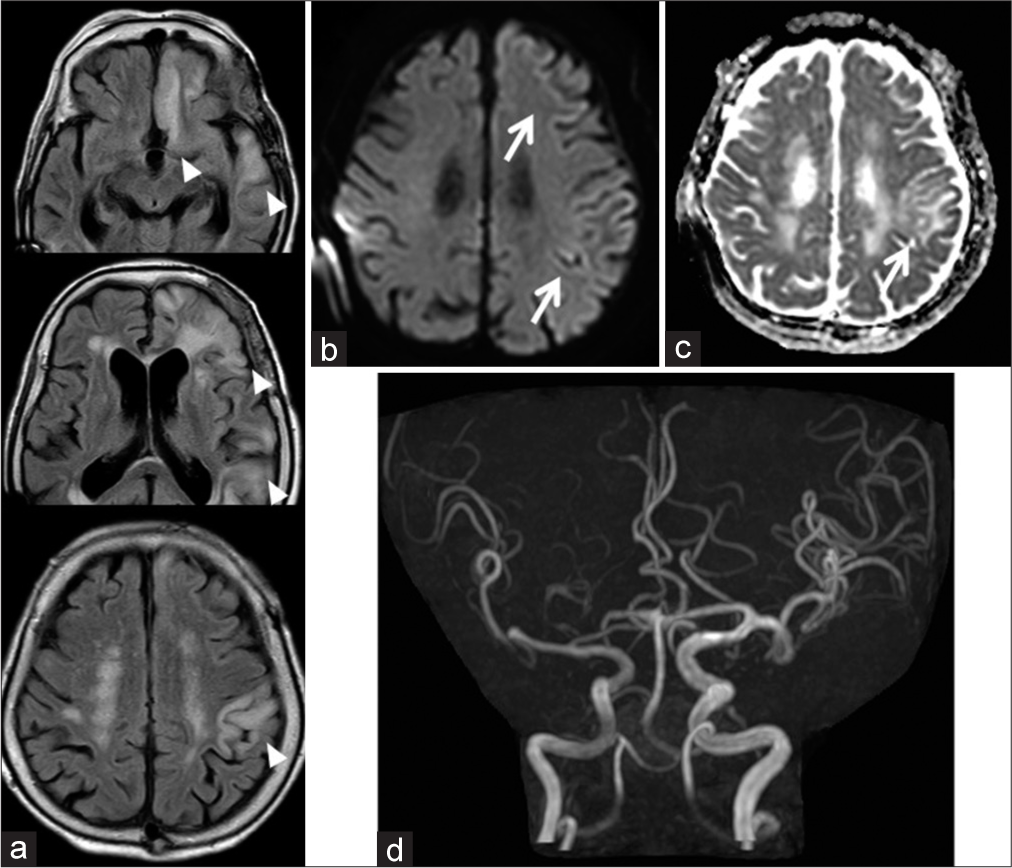
MRI findings 6 days after admission are shown as follows. (a) There are asymmetrical hyperintensity areas such as the left rectal gyrus, temporal lobe, parietal lobe, insular cortex (not shown), and thalamus (not shown) on FLAIR imaging (arrowhead). (b and c) DWI and ADC map show hyperintensity in the left cortical areas (arrow). (d) Increased signal intensity of the left MCA on MRA is revealed. DWI: Diffusion-weighted imaging, ADC: Apparent diffusion coefficient, FLAIR: Fluid-attenuated inversion recovery, MCA: Middle cerebral artery, MRA: Magnetic resonance angiography.
DISCUSSION
PRES was first reported by Hinchey et al. in 1996.[
PRES is thought to be associated with various systemic conditions such as severe hypertension, eclampsia, bacterial infections, transplantation, renal failure, and patients with autoimmune diseases receiving immunosuppressive therapy or high-dose chemotherapy.[
On average, approximately 40% of all patients diagnosed with PRES require intensive care monitoring and treatment due to severe complications such as epilepsy attack, cerebral ischemia, intracranial hemorrhage, or increased intracranial pressure.[
Recognizing the exact mechanism of PRES, there are two leading theories regarding the pathophysiology. One is the breakthrough theory that the blood–brain barrier (BBB) collapses due to impaired cerebrovascular autoregulation, which leads to subsequent cerebral edema. The other is the vasospasm theory that neurological deficits appear because of cerebral ischemia associated with cerebral vasospasm. Both theories have been confrontationally argued, but now breakthrough theory seems to be somewhat more espoused.[
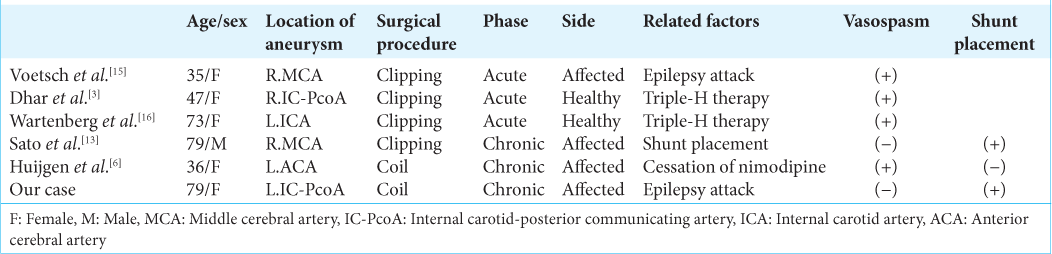
In the patients of PRES, lesions are mostly symmetric, affecting the temporal, parietal, and occipital lobes, and exhibit characteristics such as the hyperintensity area on FLAIR images and increasing ADC value without abnormal signal on DWI images. In our case, however, the lesions were asymmetric and involved the anterior lobes. Few papers have summarized cases of PRES that developed during SAH treatment, most of which lesions were bilateral.[
The differences in the affected side depending on the time of onset are interesting. In the acute phase of SAH, the combination of induced hypertension, hypervolemia, and hemodilution (triple-H therapy) is often utilized to maintain blood flow and prevent cerebral vasospasm. Consequently, cerebral blood flow is presumed to paradoxically increasing in healthy hemisphere, and it induces the breakdown of BBB. Therefore, in patients with SAH accompanied cerebral vasospasm, unilateral PRES may be more likely to occur on the healthy side. Conversely, in the chronic phase, blood flow and metabolism in the affected hemisphere that strongly suffers initial damage of SAH are easily increased by triggers such as epilepsy attack, hypertension, and hydrocephalus.
As stated above, vasogenic edema following the breakdown of BBB has been postulated as a central mechanism of PRES.[
This is how excessive elevation of blood flow in the hemisphere is inferred to lead to BBB damage and subsequent asymmetric PRES.
CONCLUSION
We report a case of asymmetric PRES secondary to epilepsy occurring in the chronic phase of SAH. If asymmetrical radiological changes were observed after SAH, even in the acute or chronic phase, we should consider this clinical condition.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Cariddi LP, Damavandi PT, Carimati F, Banfi P, Clemenzi A, Marelli M. Reversible encephalopathy syndrome (PRES) in a COVID-19 patient. J Neurol. 2020. 267: 3157-60
2. Casey SO, Sampaio RC, Michel E, Truwit CL. Posterior reversible encephalopathy syndrome: Utility of fluid-attenuated inversion recovery MR imaging in the detection of cortical and subcortical lesions. AJNR Am J Neuroradiol. 2000. 21: 1199-206
3. Dhar R, Dacey R, Human T, Zipfel G. Unilateral posterior reversible encephalopathy syndrome with hypertensive therapy of contralateral vasospasm: Case report. Neurosurgery. 2011. 69: E1176-81
4. Endo T, Niwa R, Kunii N, Matsuhashi A. A case of posterior reversible encephalopathy syndrome caused by hyperperfusion after carotid endarterectomy. Jpn J Stroke (Jananese). 2021. 43: 25-30
5. Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996. 334: 494-500
6. Huijgen W, van der Kallen B, Boiten J, Lycklama À Nijeholt G. Unilateral reversible posterior leukoencephalopathy syndrome after coiling of an aneurysm. J Clin Neurol. 2014. 10: 59-63
7. Kawakita K, Abe Y, Kirizume K, Shinohara N, Kawai Nobuyuki, Tamiya T. Posterior reversible encephalopathy syndrome (PRES): Five case reports. JJAAM (Japanese). 2012. 23: 357-63
8. Kikuchi O, Ishizaki K. A case of unilateral posterior reversible encephalopathy syndrome occurring after carotid artery stenting. JNET. 2017. 11: 403-8
9. Lee MJ, Cha J, Choi HA, Woo SY, Kim S, Wang SJ. Blood-brain barrier breakdown in reversible cerebral vasoconstriction syndrome: Implications for pathophysiology and diagnosis. Ann Neurol. 2017. 81: 454-66
10. Lee VH, Wijdicks EF, Manno EM, Rabinstein AA. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008. 65: 205-10
11. Muhammad S, Güresir Á, Greschus S, Scorzin J, Vatter H, Güresir E. Posterior reversible encephalopathy syndrome as an overlooked complication of induced hypertension for cerebral vasospasm: Systematic review and illustrative case. Stroke. 2016. 47: 519-22
12. Parauda SC, Gao V, Gewirtz AN, Parikh NS, Merkler AE, Lantos J. Posterior reversible encephalopathy syndrome in patients with COVID-19. J Neurol Sci. 2020. 416: 117019
13. Sato H, Koizumi T, Sato D, Endo S, Kato S. Unilateral posterior reversible encephalopathy syndrome after ventriculoperitoneal shunt for normal pressure hydrocephalus following subarachnoid hemorrhage: A case report. No Shinkei Geka. 2016. 44: 507-15
14. Vandenbossche G, Maquet J, Vroonen P, Lambert G, Nisolle M, Kridelka F. A reversible posterior leucoencephalopathy syndrome including blindness caused by preeclampsia. Facts Views Vis Obgyn. 2016. 8: 173-177
15. Voetsch B, Tarlov N, Nguyen TN, DeFusco C, Barest GD, Norbash A. Asymmetric posterior reversible encephalopathy syndrome complicating hemodynamic augmentation for subarachnoid hemorrhage-associated cerebral vasospasm. Neurocrit Care. 2011. 15: 542-6
16. Wartenberg KE, Parra A. CT and CT-perfusion findings of reversible leukoencephalopathy during triple-H therapy for symptomatic subarachnoid hemorrhage-related vasospasm. J Neuroimaging. 2006. 16: 170-5
17. Weidauer S, Gaa J, Sitzer M, Hefner R, Lanfermann H, Zanella FE. Posterior encephalopathy with vasospasm: MRI and angiography. J Neurol. 2003. 45: 869-76
18. Wijeratne T, Wijeratne C, Karimi L, Sales C, Crewther SG. Posterior reversible leukoencephalopathy syndrome (PRES) as a biologically predictable neurological association in severe COVID-19. First reported case from Australia and review of internationally published cases. Front Neurol. 2020. 11: 600544