- Federal Centre of Treatment and Rehabilitation of Ministry of Healthcare of Russian Federation, 125367 Moscow, Russia
Correspondence Address:
Andrey Rostislavovich Sitnikov
Federal Centre of Treatment and Rehabilitation of Ministry of Healthcare of Russian Federation, 125367 Moscow, Russia
DOI:10.4103/sni.sni_24_18
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Andrey Rostislavovich Sitnikov, Yuri Alekseevich Grigoryan, Lidiya Petrovna Mishnyakova. Awake craniotomy without sedation in treatment of patients with lesional epilepsy. 03-Sep-2018;9:177
How to cite this URL: Andrey Rostislavovich Sitnikov, Yuri Alekseevich Grigoryan, Lidiya Petrovna Mishnyakova. Awake craniotomy without sedation in treatment of patients with lesional epilepsy. 03-Sep-2018;9:177. Available from: http://surgicalneurologyint.com/surgicalint-articles/8994/
Abstract
Background:The use of awake craniotomy for surgical treatment of epilepsy was applied in surgery of convexital tumors, arteriovenous malformations, some superficial aneurysms, and stereotactic neurosurgery. The aim of this study was to show the advantages of awake craniotomy without sedation, accompanied by intraoperative neurophysiological monitoring in patients with symptomatic epilepsy.
Methods:This article describes the results of surgical treatment in 41 patients with various pathologies; 31 among them suffered from epilepsy.
Results:Most frequently, the pathological foci were located in frontal and parietal lobes nearby eloquent brain areas. Irrespective of damage location, simple partial and complex partial seizures were seen almost with the same frequency. Intraoperative mapping of eloquent cortical areas and subcortical tracts without sedation resulted in total resection of pathological area in 75% of cases with low rate of permanent neurological deficit (two patients). Minor perioperative complications, including the decrease in blood pressure in six patients and intraoperative convulsions in two patients, were handled and did not led to operation termination or anesthesia conversion. Excellent seizures control (Engel 1) was achieved in 80% of patients with available catamnesis.
Conclusion:Thus, the proposed method allows eliminating the complications associated with sedation and provides radical resection of pathological epileptogenic foci with low complication rate.
Keywords: Awake craniotomy, brain mapping, epilepsy, neurophysiological monitoring
INTRODUCTION
The use of awake craniotomy for the surgical treatment of epilepsy was introduced by V. Horsley in 1886 and subsequently applied in surgery of convexital tumors, arteriovenous malformations, some superficial aneurysms, and stereotactic neurosurgery.[
The possibilities of intraoperative brain mapping and neurophysiological monitoring, provided by awake craniotomy, allow its implementation even in pediatric patients with mental disorders.[
Currently, there are three fundamentally different approaches of performing awake craniotomy: asleep–awake–asleep (SAS) developed and modified by W. Penfield, K. Hall and D. Ingvar in 1950s; awake–awake–awake (AAA), suggested by E. Hansen in 2013; and monitored anesthesia care (MAC).[
The awake craniotomy with intraoperative neurophysiological monitoring allows improving the results of surgical treatment, to increase the extent of surgical resections at the same time minimizing the neurological complications and reducing the length of hospitalization.[
Despite the modern possibilities of functional magnetic resonance imaging (fMRI), tractography, and intraoperative navigation, only a direct contact with the patient during the intervention allows achieving better functional results.[
METHODS
The AAA craniotomy protocol was applied in our clinic from 2006. At this moment, 164 patients were operated with various pathological conditions. There were 98 men and 66 females from 18 to 77 years of age. The majority of patients presented with convexsital/superficially located lesions not exciding 3 cm. In such cases, the indication for AAA craniotomy was decreased risk of general anesthesia in comparison to the possibility of safe resection of small lesions located outside the eloquent areas with the use of a local anesthetic. The pathology was distributed as follows: meningiomas 66, glial tumors Grade I–II 35, glial tumors Grade III–IV 19, vascular abnormalities (cavernomas and small convexital arteriovenous malformations [AVMs]) 14, and metastatic lesions 30.
Among 164 patients, 41 patients (26 men and 15 women) from 19 to 74 years of age suffered from epilepsy. In those cases, the eligibility criteria for awake craniotomy were as follows: the localization of brain lesion near the suspected eloquent brain areas, the necessity of intraoperative monitoring of functions and the performance of intraoperative corticography, the need of preservation of cognitive functions, the absence of function loss at the time of surgery (hemiplegia, total aphasia), and expressed psychoemotional lability.
In addition to neurological examination, the preoperative protocol included 1.5T and 3T MRI, fMRI, and diffusion tensor imaging tractography to evaluate anatomic interrelations of the lesion with eloquent brain areas and tracts. All patients underwent a long-term electroencephalogram video monitoring to identify the source of pathological activity before and after surgical interventions.
Prior to the surgery, the patients were informed about the surgical intervention plan, the possible risks and complications of surgery, and the alleged uncomfortable sensations associated with craniotomy. The sound phenomena related to surgical intervention (sound of electrocoagulator, perforator, vacuum aspirator, and pneumatic drill) and possible inconveniences (forced position on the operating table, the probability of aphasia occurrence, or uncontrolled muscle contractions during cortical stimulation, seizure development) were described to the patients.
Fixation of the patients’ head on the operating table was performed under local anesthesia with 5 mL of 0.75% ropivacaine (Naropin) mixed with 5 mL of 1% lidocaine, which were administered in equal amounts in the region of the three-point Mayfield clamp fixation. The WarmTouch heating system was used for patients intraoperatively to maintain a comfortable body temperature in the range of 36°C–37°C and to prevent a thermoregulatory tremor.
After patient's positioning, patient registration in Medtronic StealthStation neuronavigation system, planning for trepanation, and incision were performed. The size of the planned trepanation exceeded the area of the pathological focus, determined by neuronavigation between 2 and 4 cm to perform cortical function mapping and corticography.
Planned skin incision line was infiltrated with a mixture of 0.75% ropivacaine (Naropin) and 1% lidocaine in a 1:1 ratio. If it was necessary to dissect the temporal muscle, it was infiltrated with the same solution. Since local anesthesia was achieved, a skin incision and the subsequent surgery stages were performed. Locoregional anesthesia of the nerves branches innervating the scalp and local infiltration of the dura mater with anesthetics solutions were not required in any case.
After trepanation and dura mater incision, all patients underwent corticography with Auragen™ platinum strip electrodes and grid electrodes (Integra LifeSciences). The brain area covered with electrodes was determined by the neuronavigation system and captured the area of the pathological focus and the adjacent cortical areas at a distance of not less than 1.5–2 cm from the margins of the lesion.
The choice of tests for cortical functions mapping was determined by the anatomic location of the lesion. All patients underwent bipolar biphasic stimulation with rectangular current using the cortical stimulator Ojemann (Radionics) with parameters 1 ms/phase, 60 Hz, and 2–20 mA for motor functions’ mapping. Motor response was registered on the contralateral side with subcutaneous needle electrodes placed on mm. orbiclaris oris, orbicularis oculi, masseter, trapezius, deltoid, triceps, brachioradialis, abductor policis brevis, abductor digitis minimi, quadriceps, anterior tibialis, and abductor halluces.
To identify speech centers, object naming tests and reading with a direct monopolar monophasic electrical stimulation with a stepwise current intensity increase from 2 to 11 mA under control of the intraoperative corticography were used prior to the occurrence of verbal disturbances or after discharges on electrocorticography. Evaluation of seizures control in the postoperative period was carried out according to Engel's scale and ILAE scale.
RESULTS
The tumors of different degrees of malignancy and cavernous angiomas located in eloquent brain areas were the most common lesions [
According to the preoperative MRI data, the mean volume of the pathological focus of glial tumors Grade I–II was 33.9 cm3 (from 3.71 to 70 cm3), of Grade III–IV tumors 33.4 cm3 (from 2.34 to 93.1 cm3), and of metastatic lesions 5.67 cm3 (from 0.2 to 10.9 cm3). The volume of malformations of cortical development varied from 2 to 20 cm3 (average volume 11.7 cm3), and the mean volume of cavernomas located in eloquent epileptogenic zones was 1.3 cm3. Regression analysis did not reveal any correlation between the volume of pathological zone and the extent of resection (P > 0.552).
Focal neurological deficit manifested by the moderate motor impairment was noted in four patients (three patients with glial tumors Grade III–IV and in one patient with transmantle focal cortical dysplasia of the parietal lobe). In two patients with parietal lobe lesions (one patient with glial tumor Grade IV and one with metastatic lesion), only a moderate motor deficit was noted.
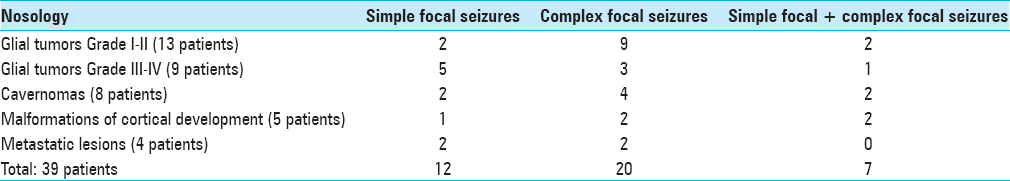
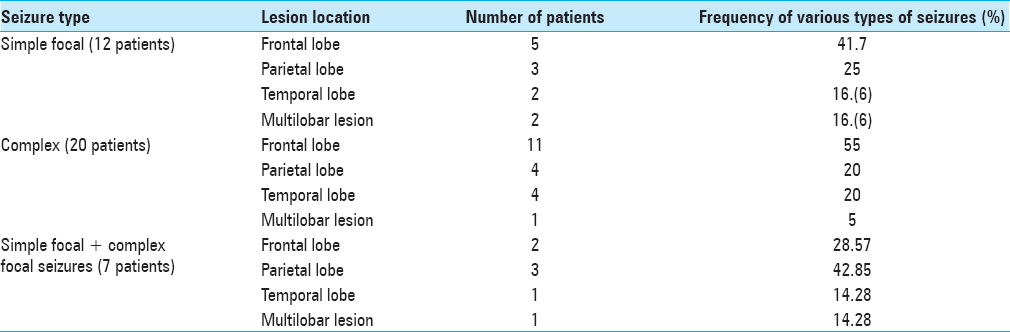
Epileptic seizures were the dominant symptom in 39 patients; among them, 12 had only focal seizures [
Irrespective of lesion location, the focal simple and the focal complex seizures were observed approximately at the same frequency without significant differences in incidence according to McNemar's test (P > 0.069) [
Fourteen patients did not receive any anticonvulsant therapy at the time of hospitalization, 20 patients received monotherapy, and 7 patients received polytherapy. The group of patients who received polytherapy presented with malformations of cortical development (three patients) and cavernomas (four patients). The main drug used as a monotherapy was carbamazepine at an average daily dose of 600–1200 mg/day. Two patients received valproic acid and two oxcarbazepine in the average therapeutic dosages. The polytherapy schemes included carbamazepine, levetiracetam, oxcarbazepine, valproic acid, and topiramate in various combinations and dosages. Long-term seizures remission was not achieved in any patient.
The average duration of surgical intervention from the moment of patient's head fixation till the wound closure was 139 min (from 69 to 207 min), and the mean amount of local anesthetic (0.75% solution of ropivacaine and 1% solution of lidocaine) was 31.2 mL.
Short-term decrease in systolic blood pressure by more than 10 mm Hg at the stage of patient's fixation due to administration of local anesthetic was seen in six patients. This complication was noted only at the stage of fixation and did not require a change in surgical plan or anesthesia tactics. After spontaneous blood pressure restoration to the initial point and repeated administration of local anesthetics for skin incision line, an additional blood pressure decrease to 90/50 mm Hg was recorded only in one patient.
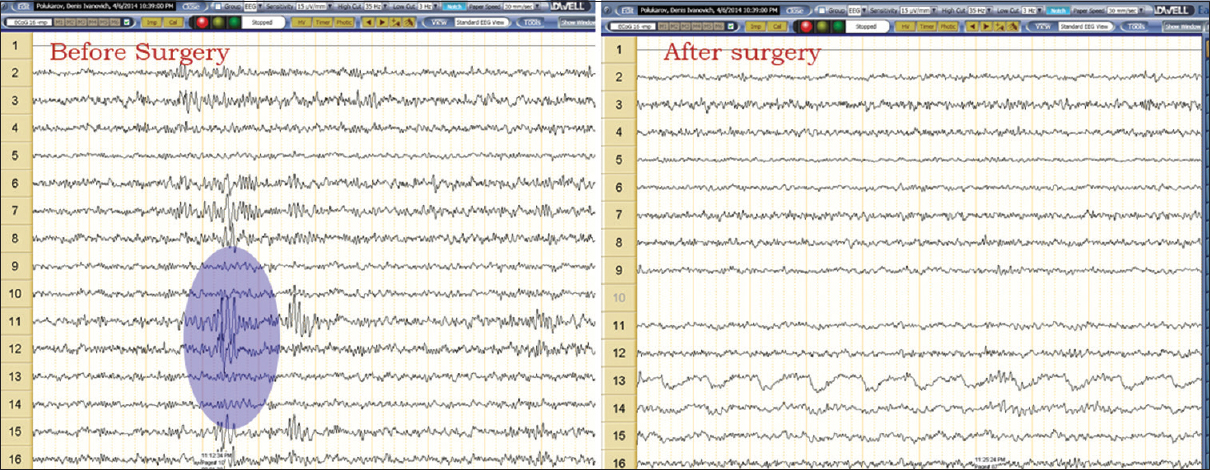
Intraoperative corticography [
In low-grade glial tumors Grade I–II and cavernomas, the primary epileptogenic zone was located at a distance of no more than 1.5–2 cm from the margins of the epileptogenic lesion and was resected in those cases when its location did not coincide with the eloquent area determined by cortical stimulation. In case of malformations of cortical development, the epileptogenic zone was partially located in the area of epileptogenic lesion, extending beyond its margins for a distance of 1–1.5 cm in three patients and was resected in all cases.
Additional resection of the primary epileptogenic zone, revealed by the results of corticography, was performed in 18 of 39 patients. Nine patients with malignant glial neoplasms (Grade III–IV), four patients with metastatic lesions, six patients with gliomas Grade I–II, and two patients with cavernomas did not undergo an additional cortical resection.
The avoidance of additional cortical resection in patients with malignant neoplasms was stipulated by normalization of the corticography pattern after lesion resection, in patients with low-grade gliomas – by the location of the epileptogenic zone in the eloquent cortical areas according to the intraoperative cortical stimulation data.
Intraoperative epileptic seizures induced by cortical stimulation were observed in two patients (4.8%) – in one of them a focal simple seizure and in one patient generalized tonic-clonic seizure. All seizures were managed by cortex irrigation with 4°C Ringer's solution and did not require anesthesia conversion or surgery termination.
Speech arrest during motor speech centers’ stimulation was noted in 18 of 27 patients with localization of the pathological process in the left hemisphere and in two patients in the right hemisphere lesion, whose speech centers were located in the right hemisphere according to fMRI data (an intraoperative video is available at
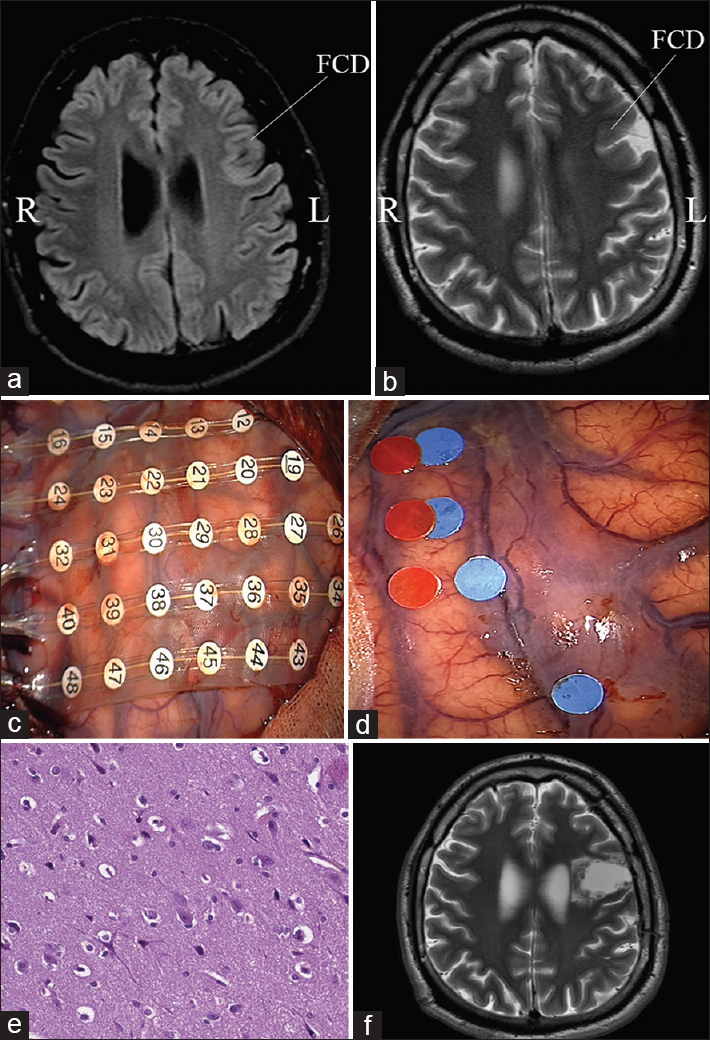
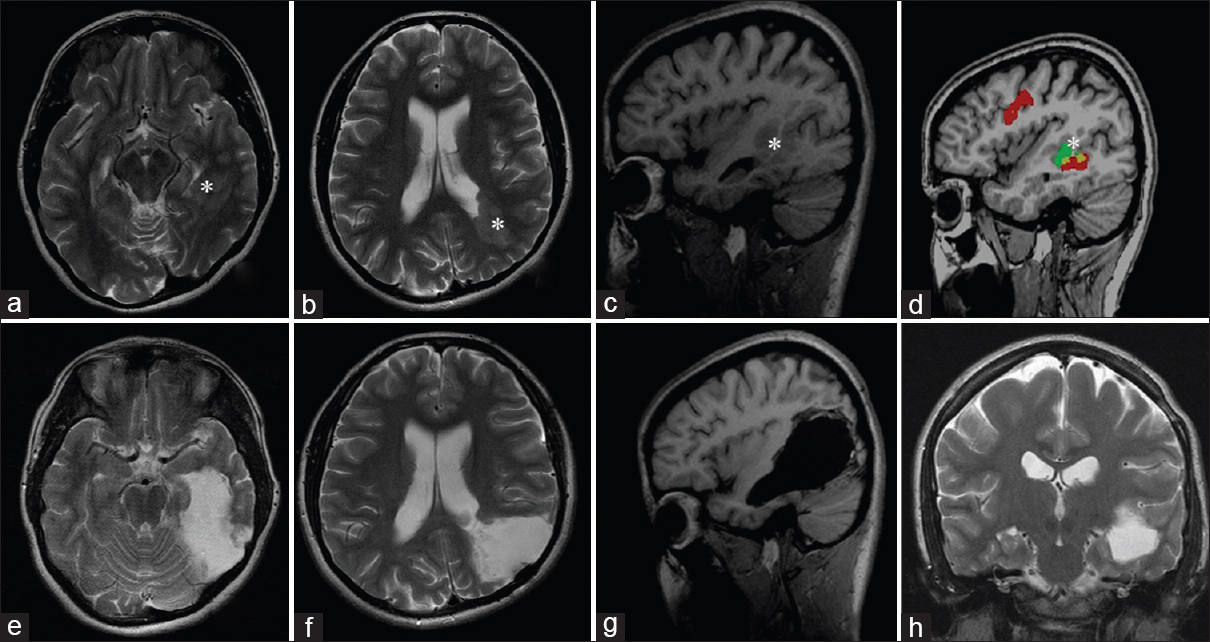
Figure 2
Resection of left frontal focal cortical dysplasia with intraoperative corticography and direct cortical stimulation. (a and b) Preoperative magnetic resonance study, the area of dysplasia is marked with an arrow; (c) location of ECoG electrodes above the projection of focal cortical dysplasia and adjacent cortical areas; (d) the results of determining the source of epileptic activity (the corresponding labels are marked with blue dots) and the speech arrest area (the labels are marked with red dots); (e) patomorphological specimen demonstrates typical features of focal cortical dysplasia; (f) postoperative magnetic resonance imaging after total focal cortical dysplasia resection)
Motor response during direct cortical stimulation of primary motor centers was obtained in 36 of 39 patients. The use of intraoperative mapping of the eloquent areas allowed the total removal of patholocical foci in 31 patients (75%) [
Figure 3
Patient's magnetic resonance imaging image before and 3 months after the total removal of left-sided periventricular gray matter heterotopia extending from occipital to temporal lobe using the awake–awake–awake craniotomy ([a–d] preoperative magnetic resonance imaging; [e–h] postoperative images). Heterotopia marked with white asterisk. Image “d” demonstrates the data obtained from functional magnetic resonance imaging showing the close location of sensory speech area to heterotopia
In all cases of partial and subtotal removal, resection was stopped due to development of motor deficiency or verbal disturbances in patients. New neurological deficit after surgery [
The aphasia was a prevailing symptom among all additional neurological disorders and in majority of cases was associated with damage of arcuate fasciculus. Permanent neurological deficit associated with internal capsule damage was developed in two patients – in one case with a partially removed giant oligodendroglioma spreading into the posterior limb of the internal capsule and in one case with total removal of the temporoinsular glioblastoma.
In the majority of cases, the development of new neurological deficit during the surgery was observed in patients with malignant tumors (gliomas Grade III–IV and metastatic lesions extended beyond one lobe) due to their hypervascularity compared with benign tumors and congenital malformations of cortical development and, therefore, technical difficulties in achieving adequate hemostasis when leaving even the small fragments of tumor tissue. No other serious intraoperative and early postoperative complications were recorded.
Postsurgical evaluation in terms of seizure control was carried out in the groups of patients with cavernomas, malformations of cortical development, and benign gliomas. Catamnesis in patients with metastatic lesions and high-grade gliomas was not evaluated because the majority of patients in these groups did not survive till the control point (12 months) as a result of natural progression of the disease.
Among 26 patients whose anamnestic data were valid, 21 patients were completely seizure-free or have rare partial seizures (ILAE class 1a, grade 2; Engel I) and 5 patients had rare seizures (ILAE class 3, Engel Ic).
DISCUSSION
The use of awake craniotomy in surgical treatment of patients with symptomatic epilepsy caused by various brain lesions provides a number of unique advantages, including the possibility of intraoperative evaluation of the clinical and neurological status, a reduction in postoperative neurologic complications and reduction in hospitalization.[
The choice of awake craniotomy approach is primarily determined by the possibility of adequate control of neurological and vital functions during the surgery and by possible complications associated with the use of the method. Complications occurred during awake craniotomy can be divided into two groups: anesthesia-related and surgery-related. The first group includes the obstruction of the upper respiratory tract, hypoxia, conversion to general anesthesia, hypertension (hypotension), tachycardia (bradycardia), nausea (vomiting), toxic effects of local anesthetic, pain, poor patient cooperation, and agitation. The surgical complications can be presented with focal seizures, generalized seizures, the appearance of a neurological deficit (aphasia, paresis), bleeding, cerebral edema, and air embolism.[
According to meta-analysis of literature published between 2007 and 2015, which included the results of 5945 awake craniotomies in 5931 patients using all three approaches, in the group of patients who underwent craniotomy under the SAS or MAC protocols, the frequency of the most common complications was distributed as follows: intraoperative seizures 8%, new neurological deficit 17%, and the conversion to general anesthesia 2%.[
Among the complications that lead to the impossibility of further evaluation of neurological functions using the SAS and monitored anesthetic management protocols, there are a laryngeal mask leak, respiratory failure, intraoperative bleeding, intraoperative anxiety and pain, cerebral edema, convulsive seizures, and air embolism.[
Nossek et al. analyzed 477 interventions in their study under “MAC” protocol (remifentanil + propofol in low doses in the beginning and in the end of surgical intervention with the absence of medication during the awake phase); the incidence of intraoperative seizures reached 12.6%, while in 37 patients surgical intervention became impossible due to complications and in 7 patients the development of seizures led to conversion to general anesthesia.[
The frequent drugs used for awake craniotomy are propofol, remifentanil, dexmedetomidine, and their combinations.[
The main advantage of dexmedetomidine is the ability to keep the epileptic activity unchanged, which allows to use it while performing intraoperative corticography in cases of epilepsy and epileptogenic lesions.[
Despite the publications claiming the “harmlessness” of dexmedetomidine use in neurosurgical practice, a combined report of the US Department of Health and Human Services and the Office of Food and Drug Administration (FDA) published on 03/10/2016 contains 37 reports of the various side effects caused by the use of this drug, most often in pediatric practice. A fatal hypotension and bradycardia, prolonged QT syndrome, fulminant hepatitis, acute adrenal insufficiency, and encephalopathy are listed among the complications.[
A detailed analysis of the latest publications on the awake craniotomy issue makes doubtful the generally accepted view on the absolute and undeniable safety and validity of the implementation of the “SAS” and “MAC” protocols in neurosurgical practice.
On this background, E. Hansen's publication dedicated to awake craniotomy without sedation suggests a different way to address the issues related to the use of medications.[
The issue of the use of locoregional anesthesia and local scalp anesthesia at the incision site has also been widely discussed in literature. Mostly, the authors use a combination of locoregional anesthesia with local anesthesia of the scalp incision area using various combinations of local anesthetics. The performed meta-analysis confirmed the efficacy of anesthesia of the nerves branches innervating the scalp, including the achievement of postoperative analgesia.[
The average duration of surgical interventions in E. Hansen's series was 217 ± 45 min (105–295 min), which is also similar to our data. Considering that onset of anesthesia and its duration with conduction and infiltration anesthesia with a 0.75% solution of ropivacaine are 1–15 min and 2–6 h, respectively, the patients do not experience significant pain during the main stages of the surgery. The main painful sensations were commonly associated with locoregional anesthesia (in our series anesthesia of scalp incision) and less often a surgical wound suturing.
The incidence of intraoperative seizures in E. Hansen's patient group is significantly higher than the average data. Seizures were noted in eight patients (16%), in five patients focal and three generalized. In one case, the presence of seizures led to the conversion of the procedure to general anesthesia. In our series, focal convulsive seizures were observed in only two patients. Such a difference may be predetermined by the different protocols of cortical stimulation, which, unfortunately, cannot be analyzed in E. Hansen's work.
A high incidence of intraoperative seizures and a fairly high number of neurological complications (2% severe neurological deficit and 14% moderate neurological deficit) in a series published by E. Hansen served as one of the cornerstones of criticism of the method. In his commentary to the article, H. Duffau pointed out that the use of the SAS protocol is more correct, since it has a low complication rate (2% of permanent neurologic deficits). In his own series of 140 cases, the patients had no seizures, cerebral edema, severe neurologic deficits, and only one case of aspiration.[
Arguments made by H. Duffau in favor of the use of the “SAS” protocol consisted of “the possibility of saving the patient from the horrific sound during craniotomy,” “the complexity of re-operating in glioma relapses due to the adhesion process,” and “the need for manipulation on vessels in large gliomas, for example in frontotemporal-insular localization.” In our opinion, these provisions are more subjective and undoubtedly mitigated by the possible complications that inevitably arise if the patient is sedated.
CONCLUSION
Our study demonstrates the possibility of the safe use of awake craniotomy without sedation in patients with brain lesions accompanied by symptomatic epilepsy. The undeniable advantages of the suggested approach are the elimination of possible complications associated with the use of general anesthetics, trachea intubation, and the possibility of adequate neurophysiological monitoring, which is not affected by drugs that are routinely used for awake craniotomy under SAS and MAC protocols.
Awake craniotomy without sedation is a reproducible technique that is easily tolerated by patients when meeting the selection criteria and the rules of preoperative preparation, and allows to expand the possibilities of surgical treatment of lesions located in the eloquent areas.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alimohamadi M, Shirani M, Shariat Moharari R, Pour-Rashidi A, Ketabchi M, Khajavi M. Application of awake craniotomy and intraoperative brain mapping for surgical resection of insular gliomas of the dominant hemisphere. World Neurosurg. 2016. 92: 151-8
2. Andersen JH, Olsen KS. Anaesthesia for awake craniotomy is safe and well-tolerated. Dan Med Bull. 2010. 57: A4194-
3. Bernstein M. Outpatient craniotomy for brain tumor: A pilot feasibility study in 46 patients. Can J Neurol Sci. 2001. 28: 120-4
4. Blanshard HJ, Chung F, Manninen PH, Taylor MD, Bernstein M. Awake craniotomy for removal of intracranial tumor: Considerations for early discharge. Anesth Analg. 2001. 92: 89-94
5. Burchiel KJ, Clarke H, Ojemann GA, Dacey RG, Winn HR. Use of stimulation mapping and corticography in the excision of arteriovenous malformations in sensorimotor and language-related neocortex. Neurosurgery. 1989. 24: 322-7
6. Chacko AG, Thomas SG, Babu KS, Daniel RT, Chacko G, Prabhu K. Awake craniotomy and electrophysiological mapping for eloquent area tumours. Clin Neurol Neurosurg. 2013. 115: 329-34
7. Duffau H, Capelle L, Sichez J, Faillot T, Abdennour L, Law Koune JD. Intra-operative direct electrical stimulations of the central nervous system: The salpêtrière experience with 60 patients. Acta Neurochir (Wien). 1999. 141: 1157-67
8. Duffau H. The reliability of asleep-awake-asleep protocol for intraoperative functional mapping and cognitive monitoring in glioma surgery. Acta Neurochir (Wien). 2013. 155: 1803-4
9. Ghazanwy M, Chakrabarti R, Tewari A, Sinha A. Awake craniotomy: A qualitative review and future challenges. Saudi J Anaesth. 2014. 8: 529-39
10. Grossman R, Ram Z. Awake craniotomy in glioma surgery. Eur Assoc Neurol Oncol Mag. 2014. 4: 27-33
11. Guilfoyle MR, Helmy A, Duane D, Hutchinson PJ. Regional scalp block for postcraniotomy analgesia: A systematic review and meta-analysis. Anesth Analg. 2013. 116: 1093-102
12. Gupta DK, Chandra PS, Ojha BK, Sharma BS, Mahapatra AK, Mehta VS. Awake craniotomy versus surgery under general anesthesia for resection of intrinsic lesions of eloquent cortex – A prospective randomised study. Clin Neurol Neurosurg. 2007. 109: 335-43
13. Hall K, Baldwin M, Norris F Jr. Succinylcholine drip during craniotomy. Anesthesiology. 1959. 20: 65-70
14. Hansen E, Seemann M, Zech N, Doenitz C, Luerding R, Brawanski A. Awake craniotomies without any sedation: The awake-awake-awake technique. Acta Neurochir (Wien). 2013. 155: 1417-24
15. Hervey-Jumper SL, Li J, Lau D, Molinaro AM, Perry DW, Meng L. Awake craniotomy to maximize glioma resection: Methods and technical nuances over a 27-year period. J Neurosurg. 2015. 123: 325-39
16. Hill CS, Severgnini F, McKintosh E. How I do it: Awake craniotomy. Acta Neurochir (Wien). 2017. 159: 173-6
17. Horsley V. Remarks on ten consecutive cases of operations upon the brain and cranial cavity to illustrate the details and safety of the method employed. Br Med J. 1887. 1: 863-5
18. Ingvar DH, Jeppsson ST, Nordstrom L. A new form of anaesthesia in surgical treatment of focal epilepsy. Acta Anaesthesiol Scand. 1959. 3: 111-21
19. Kulikov A, Rylova A, Lubnin A. Awake craniotomy under xenon anesthesia. J Neurosurg Anesthesiol. 2012. 24: 165-6
20. Kulikova AS, Sel’kov DA, Kobyakov GL, Shmigel’skiy AV, Lubnin AY. Awake craniotomy: In search for optimal sedation. Anesteziol Reanimatol. 2015. 60: 4-8
21. Li T, Bai H, Wang G, Wang W, Lin J, Gao H. Glioma localization and excision using direct electrical stimulation for language mapping during awake surgery. Exp Ther Med. 2015. 9: 1962-6
22. Lubnin AY, Melikian AG, Kazarian AA, Salova EM. Awake craniotomy in nine-year old girl with severe epilepsy and mental disorders. Vopr Neirokhir Im N N Burdenko. 2009. 3: 47-53
23. Lüders JC, Steinmetz MP, Mayberg MR. Awake craniotomy for microsurgical obliteration of mycotic aneurysms: Technical report of three cases. Neurosurgery. 2005. 56: E201-
24. Mahmoud M, Sadhasivam S, Salisbury S, Nick TG, Schnell B, Sestokas AK. Susceptibility of transcranial electric motor-evoked potentials to varying targeted blood levels of dexmedetomidine during spine surgery. Anesthesiology. 2010. 112: 1364-73
25. McNicholas E, Bilotta F, Titi L, Chandler J, Rosa G, Koht A. Transient facial nerve palsy after auriculotemporal nerve block in awake craniotomy patients. A A Case Rep. 2014. 2: 40-3
26. Nelson LE, Lu J, Guo T, Saper CB, Franks NP, Maze M. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003. 98: 428-36
27. Nossek E, Matot I, Shahar T, Barzilai O, Rapoport Y, Gonen T. Intraoperative seizures during awake craniotomy: Incidence and consequences: Analysis of 477 patients. Neurosurgery. 2013. 73: 135-40
28. Osborn I, Sebeo J. “Scalp block” during craniotomy: A classic technique revisited. J Neurosurg Anesthesiol. 2010. 22: 187-94
29. Penfield W. Combined regional and general anesthesia for craniotomy and cortical exploration. Anesth Analg. 1954. 33: 145-55
30. Peruzzi P, Bergese SD, Viloria A, Puente EG, Abdel-Rasoul M, Chiocca EA. A retrospective cohort-matched comparison of conscious sedation versus general anesthesia for supratentorial glioma resection. Clinical article. J Neurosurg. 2011. 114: 633-9
31. Pillai S, Hoehn K, Brouillette G. Posterior Reversible encephalopathy syndrome as a result of withdrawal from prolonged dexmedetomidine. J Pediatr Intensive Care. 2015. 4: 162-5
32. Shen SL, Zheng JY, Zhang J, Wang WY, Jin T, Zhu J. Comparison of dexmedetomidine and propofol for conscious sedation in awake craniotomy: A prospective, double-blind, randomized, and controlled clinical trial. Ann Pharmacother. 2013. 47: 1391-9
33. Shinoura N, Midorikawa A, Yamada R, Hana T, Saito A, Hiromitsu K. Awake craniotomy for brain lesions within and near the primary motor area: A retrospective analysis of factors associated with worsened paresis in 102 consecutive patients. Surg Neurol Int. 2013. 4: 149-
34. Sidhu A, Murgahayah T, Narayanan V, Chandran H, Waran V. Electroacupuncture-assisted craniotomy on an awake patient. J Acupunct Meridian Stud. 2017. 10: 45-8
35. Souter MJ, Rozet I, Ojemann JG, Souter KJ, Holmes MD, Lee L. Dexmedetomidine sedation during awake craniotomy for seizure resection: Effects on electrocorticography. J Neurosurg Anesthesiol. 2007. 19: 38-44
36. Stevanovic A, Rossaint R, Veldeman M, Bilotta F, Coburn M. Anaesthesia management for awake craniotomy: Systematic review and meta-analysis. PLoS One. 2016. 11: e0156448-