- Department of Neurosurgery, Kyoto Prefectural University Graduate School of Medical Science, Kyoto, Japan.
Correspondence Address:
Yoshinobu Takahashi, Department of Neurosurgery, Kyoto Prefectural University Graduate School of Medical Science, Kawaramachi Hirokoji, Kyoto, Japan.
DOI:10.25259/SNI_610_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Saki Kotani, Yoshinobu Takahashi, Tamaki Morisako, Takumi Yamanaka, Naoya Hashimoito. Bacterial meningitis caused by nontraumatic cerebrospinal fluid rhinorrhea with aqueductal stenosis: A case report. 23-Sep-2022;13:439
How to cite this URL: Saki Kotani, Yoshinobu Takahashi, Tamaki Morisako, Takumi Yamanaka, Naoya Hashimoito. Bacterial meningitis caused by nontraumatic cerebrospinal fluid rhinorrhea with aqueductal stenosis: A case report. 23-Sep-2022;13:439. Available from: https://surgicalneurologyint.com/surgicalint-articles/11879/
Abstract
Background: Nontraumatic cerebrospinal fluid (CSF) rhinorrhea associated with aqueductal stenosis is rare. The resulting CSF leakage may cause bacterial meningitis, and appropriately timed surgical treatment should be considered.
Case Description: A 28-year-old woman with obstructive hydrocephalus secondary to aqueductal stenosis presented with intermittent nasal discharge. CSF rhinorrhea was suspected, but she refused surgery. During the course of conservative treatment, she developed meningitis. Exacerbation of hydrocephalus and CSF rhinorrhea was suspected, and the patient underwent endoscopic third ventriculostomy after recovery from meningitis. Postoperatively, ventricular size decreased and CSF leakage completely resolved. There was no recurrence of hydrocephalus or rhinorrhea.
Conclusion: Patients with intermittent CSF rhinorrhea due to exacerbation of hydrocephalus are at high risk for bacterial meningitis. Appropriately timed surgical treatment results in a favorable outcome.
Keywords: Aqueductal stenosis, Bacterial meningitis, Cerebrospinal fluid rhinorrhea, Endoscopic third ventriculostomy, Hydrocephalus
INTRODUCTION
Cerebrospinal fluid (CSF) rhinorrhea most commonly occurs following trauma or surgery, whereas nontraumatic CSF rhinorrhea associated with aqueductal stenosis is rare. Chronically increased intracranial pressure (ICP) can lead to the formation of a CSF fistula. The resulting CSF leakage may cause bacterial meningitis, and appropriately timed surgical treatment should be considered. Closure of the CSF fistula and CSF diversion may be effective, but the optimal treatment has not been established. Here, we report an adult case of bacterial meningitis secondary to nontraumatic CSF rhinorrhea with aqueductal stenosis.
CASE REPORT
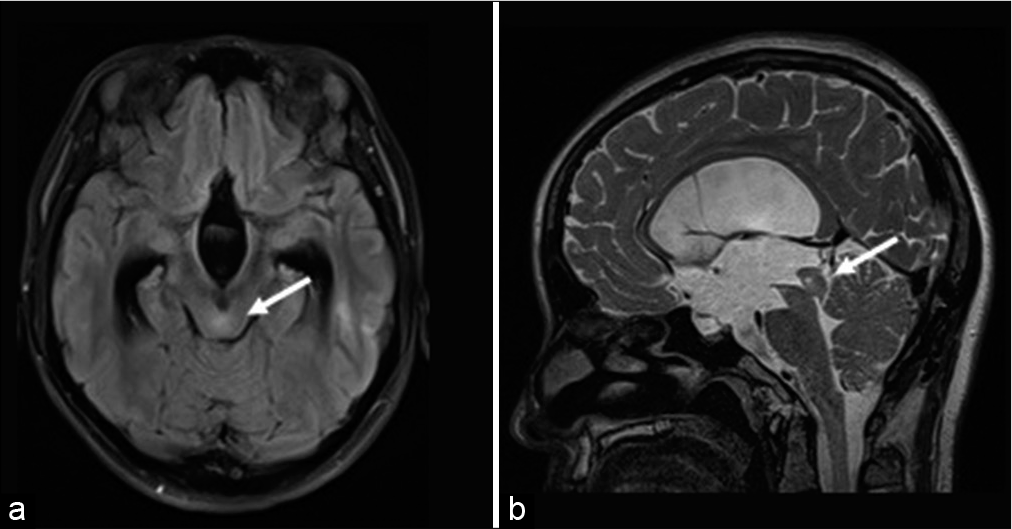
A 28-year-old woman presented with headache and intermittent watery discharge from the left nostril. She had no history of head injury, head surgery, or obesity. Magnetic resonance imaging (MRI) of the head revealed obstructive hydrocephalus with aqueductal stenosis, ventricular dilation with ballooning of the floor of the third ventricle, and CSF accumulation in the left sphenoid sinus and posterior ethmoid sinus. T2-weighted and fluid-attenuated inversion recovery (FLAIR) MRI revealed a hyperintense lesion in the tectal plate suggestive of a tectal glioma [
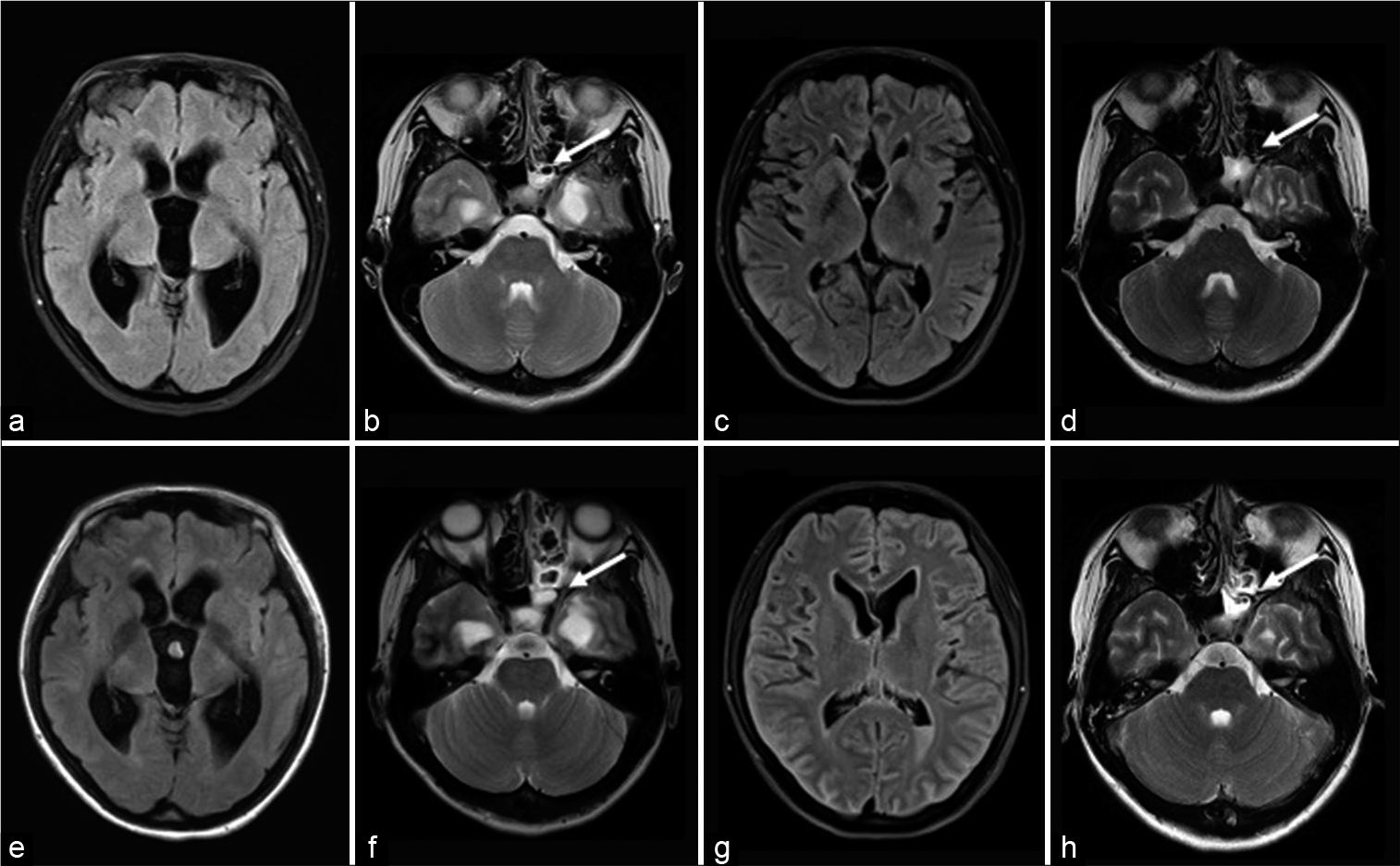
One day during follow-up in the outpatient clinic, she visited the emergency room due to headache and vomiting. She was initially alert and her body temperature was 37.2°C, but her level of consciousness deteriorated (Glasgow Coma Scale score: 5 [E2V2M1]) and body temperature increased to 39.4°C soon after admission. Nuchal rigidity was also observed. Laboratory findings showed a marked increase in WBC count (2.01 × 103/µL) and neutrophil count (92.6%). CSF analysis revealed a markedly elevated cell count (20,310/µL), elevated protein level (2 mg/dL), and decreased CSF glucose (<5 mg/dL, serum glucose 123 mg/dL). CSF pressure was 9 cmH2O. FLAIR MRI showed a hyperintense cerebral sulcus and hyperintense intraventricular lesions relative to CSF, suggesting meningitis and ventriculitis. T2-weighted MRI revealed increased CSF accumulation in the left sphenoid sinus and posterior ethmoid sinus. CSF pressure on admission was not high and there was no ventricular enlargement, but repeated ventricular enlargement and shrinkage had been observed over the past 2 years [
After recovery from meningitis, endoscopic third ventriculostomy (ETV) was performed for obstructive hydrocephalus with aqueductal stenosis. Biopsy of the tectal plate lesion was not performed because a tumor could not be identified during surgery. CSF leakage completely resolved after surgery [
DISCUSSION
We encountered a case of bacterial meningitis resulting from nontraumatic CSF rhinorrhea with obstructive hydrocephalus secondary to aqueductal stenosis. The course of the patient suggested two important clinical lessons, as follows. First, chronically increased ICP can cause CSF rhinorrhea, and hydrocephalus has repeated remission and exacerbation according to CSF stagnation and leakage. Second, ETV can be effective for treating high-pressure CSF leakage and obstructive hydrocephalus with aqueductal stenosis.
Chronically increased ICP can cause CSF rhinorrhea. Thomson provided the first clear description and definition of spontaneous CSF rhinorrhea in 1899 and attempted to distinguish this condition from that caused by trauma.[
CSF fistulas commonly occur in anatomically fragile areas such as the ethmoid sinus, cribriform plate, and sphenoid sinus.[
ETV can be effective for treating high-pressure CSF leakage and obstructive hydrocephalus with aqueductal stenosis. Management of CSF rhinorrhea is variable and has not been clearly established [
CONCLUSION
We report a case of bacterial meningitis secondary to nontraumatic CSF rhinorrhea with hydrocephalus, in which a favorable outcome was achieved with ETV. Chronically increased ICP can cause CSF rhinorrhea, which may lead to improvement in hydrocephalus when ICP decreases with increased CSF leakage. Appropriately scheduled surgery is necessary due to the high risk of meningitis. ETV can also be an effective treatment approach in cases of high-pressure CSF leakage and obstructive hydrocephalus with aqueductal stenosis.
Declaration of the patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
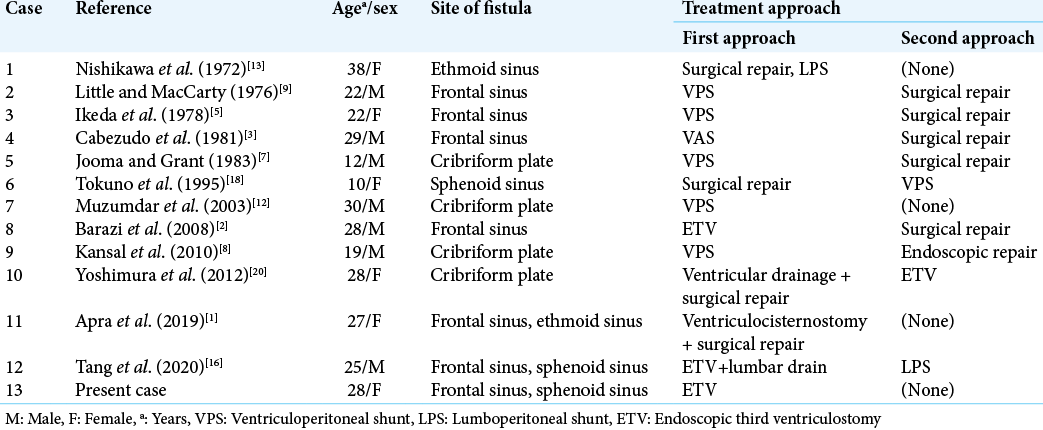
1. Apra C, Penet N, Froelich S. Cerebrospinal fluid fistula in the frontal sinus secondary to obstructive hydrocephalus. World Neurosurg. 2019. 131: 19-20
2. Barazi S, Choo M, Martin A. CSF rhinorrhea due to a pulsion diverticulum of the frontal horn. Br J Neurosurg. 2008. 22: 580-1
3. Cabezudo JM, Vaquero J, García-de-Sola R, Areitio E, Martinez R. Direct communication between the lateral ventricle and the frontal sinus as the cause of CSF rhinorrhea in aqueductal stenosis. Acta Neurochir (Wien). 1981. 57: 95-8
4. Carrau RL, Snyderman CH, Kassam AB. The management of cerebrospinal fluid leaks in patients at risk for high-pressure hydrocephalus. Laryngoscope. 2005. 115: 205-12
5. Ikeda K, Nakano M, Tani E. Tension pneumocephalus complicating ventriculoperitoneal shunt for cerebrospinal fluid rhinorrhoea: Case report. J Neurol Neurosurg Psychiatry. 1978. 41: 319-22
6. Ishiwata Y, Shinozawa T, Ichikawa T, Kojima Y, Inada Y. Efficacy of percutaneous lumboperitonial shunt for management of cerebrospinal fluid leakage. Neurol Med Chir (Tokyo). 1985. 25: 923-7
7. Jooma R, Grant DN. Cerebrospinal fluid rhinorrhea and intraventricular pneumocephalus due to iPntermittent shunt obstruction. Surg Neurol. 1983. 20: 231-4
8. Kansal R, Mahore A, Goel A. Cerebrospinal fluid rhinorrhea after ventriculoperitoneal shunt in a patient with tectal plate glioma. J Clin Neurosci. 2010. 17: 532-3
9. Little JR, MacCarty CS. Tension pneumocephalus after insertion of ventriculoperitoneal shunt for aqueductal stenosis. J Neurosurg. 1976. 44: 383-5
10. Loew F, Pertuiset B, Chaumier EE, Jaksche H. Traumatic, spontaneous and postoperative CSF rhinorrhea. Adv Tech Stand Neurosurg. 1984. 11: 169-207
11. Martínez-Capoccioni G, Serramito-García R, MartínBailón M, García-Allut A, Martín-Martín C. Spontaneous cerebrospinal fluid leaks in the anterior skull base secondary to idiopathic intracranial hypertension. Eur Arch Otorhinolaryngol. 2017. 274: 2175-81
12. Muzumdar D, Nadkarni T, Goel A. Spontaneous cerebrospinal fluid rhinorrhea as a presenting symptom of aqueductal stenosis case report. Neurol Med Chir (Tokyo). 2003. 43: 626-9
13. Nishikawa M, Karasawa J, Kamada K. Non traumatic CSF rhinorrhea associated with obstruction of the aqueduct. Brain and Nerve (Tokyo). 1972. 24: 173-7
14. Ommaya AK, Di Chiro G, Baldwin M, Pennybacker JB. Non-traumatic cerebrospinal fluid rhinorrhoea. J Neurol Neurosurg Psychiatry. 1968. 31: 214-25
15. Shetty PG, Shroff MM, Fatterpekar GM, Sahani DV, Kirtane MV. A retrospective analysis of spontaneous sphenoid sinus fistula: MR and CT findings. AJNR Am J Neuroradiol. 2000. 21: 337-42
16. Tang C, Zhu J, Feng K, Yang J, Cong Z, Cai X. Successful treatment of spontaneous cerebrospinal fluid rhinorrhea with endoscopic third ventriculostomy and lumboperitoneal shunt: A case report. Front Neurosci. 2020. 14: 57
17. Thomson St. C.editors. The Cerebro-Spinal Fluid: Its Spontaneous Escape from the Nose. London: Cassell; 1899. p.
18. Tokuno T, Ban S, Nakazawa K, Yoshida S, Matsumoto S, Shingu T. Non-traumatic cerebrospinal fluid rhinorrhea associated with hydrocephalus: A case report. No Shinkei Geka. 1995. 23: 265-9
19. Wise SK, Schlosser RJ. Evaluation of spontaneous nasal cerebrospinal fluid leaks. Curr Opin Otolaryngol Head Neck Surg. 2007. 15: 28-34
20. Yoshimura M, Matsusaka Y, Terada A, Ishiguro T, Nakajima H, Yamanaka K. Spontaneous cerebrospinal fluid rhinorrhea associated with long-standing overt ventriculomegaly in adults (LOVA). No Shinkei Geka. 2012. 40: 897-902