- College of Medicine, Gulf Medical University, Ajman, United Arab Emirates
- Department of Neurosurgery, HMS Al Garhoud Hospital, Dubai, United Arab Emirates.
Correspondence Address:
Maliya Delawan, College of Medicine, Gulf Medical University, Ajman, United Arab Emirates.
DOI:10.25259/SNI_9_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Maliya Delawan1, Abdulla Qassim2. Behavioral disinhibition following corpus callosotomy done for colloid cyst excision in 15-year-old girl: A case report and literature review. 10-Feb-2023;14:48
How to cite this URL: Maliya Delawan1, Abdulla Qassim2. Behavioral disinhibition following corpus callosotomy done for colloid cyst excision in 15-year-old girl: A case report and literature review. 10-Feb-2023;14:48. Available from: https://surgicalneurologyint.com/surgicalint-articles/12150/
Abstract
Background: There is a growing body of literature suggesting that the corpus callosum plays an important role in behavior. While behavioral deficits are a rare complication following callosotomy, they are well-documented in agenesis of the corpus callosum (AgCC), with emerging evidence reporting disinhibition among children with AgCC.
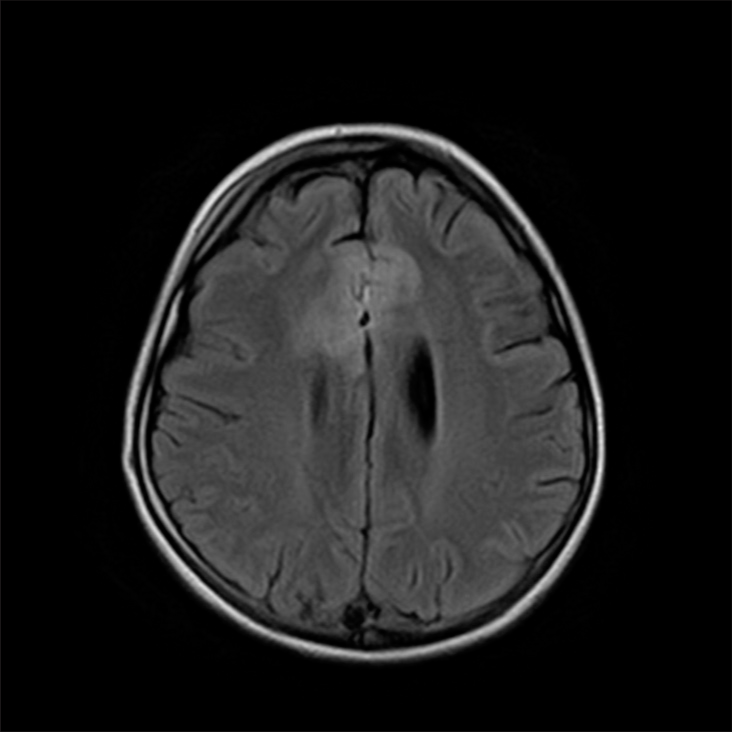
Case Description: A 15-year-old girl had undergone a right frontal craniotomy and excision of a third ventricle colloid cyst using the transcallosal approach. Ten days after the operation, she was readmitted for progressive symptoms of behavioral disinhibition. Postoperative magnetic resonance imaging of the brain showed mild-to-moderate bilateral edematous changes along the operative bed, with no other significant findings.
Conclusion: To the best of the authors’ knowledge, this is the first report in literature to describe behavioral disinhibition occurring as a sequelae to a surgical procedure involving callosotomy.
Keywords: Behavioral disinhibition, Corpus callosotomy, Corpus callosum
INTRODUCTION
The corpus callosum consists of white matter tracts that connect the right and left cerebral hemispheres. It is composed of five parts from anterior to posterior: the rostrum, genu, body, isthmus, and splenium. Fibers of the rostrum and genu arch anteriorly to form the forceps minor, interconnecting the anterior parts of the two frontal lobes. The body fibers interconnect the corona radiata of each side and other white matter pathways of the posterior part of the frontal lobe, the entire parietal lobe and the superior part of the temporal lobe. Finally, fibers of the splenium arch posteriorly and form the forceps major, interconnecting the occipital lobes of each side.[
Significant advances have been made in understanding the function of the corpus callosum by studying callosotomy patients. Observations from patients who undergo callosotomy for the treatment of epilepsy revealed topographical organization of the corpus callosum, with its anterior regions involved in the transfer of motor and high-level cognitive signals and its posterior regions involved in the transfer of somatosensory (posterior midbody), auditory (isthmus), and visual (splenium) information.[
Compared to adults, children seem to experience fewer neurologic complications following callosotomy. This could be attributed to enhanced neural plasticity in children that may allow development of alternate neural pathways.[
Individuals with agenesis of the corpus callosum (AgCC) also present a unique opportunity to learn about the role of the corpus callosum in cognitive processing. AgCC has been shown to be associated with impairment in multiple cognitive domains, most particularly in problem solving skills and processing speed. Secondary to these core deficits, individuals with AgCC may present with impairment in abstract reasoning and second-order linguistic deficits, as well as social and behavioral abnormalities.[
To the best of the authors’ knowledge, there is no published report of behavioral disinhibition following callosotomy and hence, we thought that it was worthwhile to describe this patient.
CASE DESCRIPTION
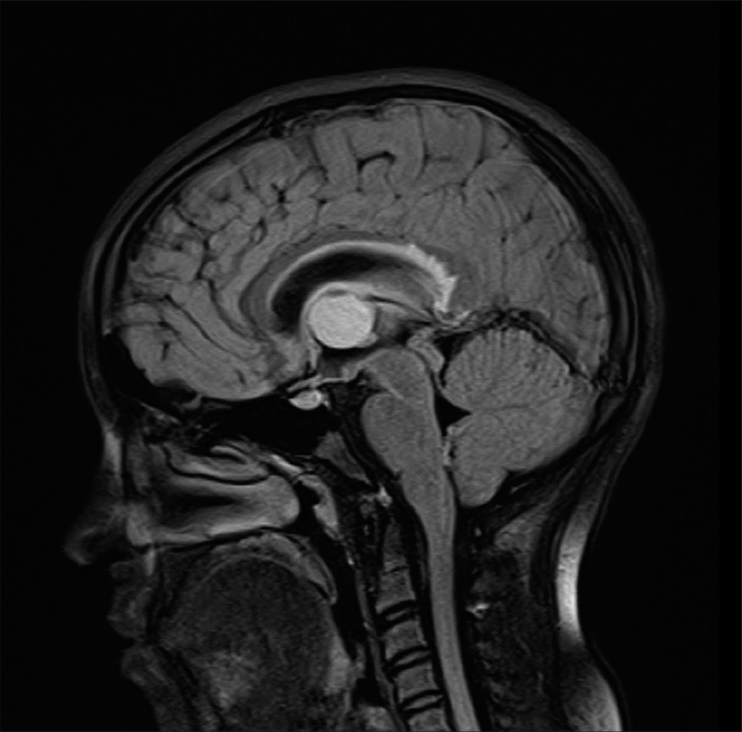
This patient was a 15-year-old girl who presented with a short history of progressive headache, dizziness, diplopia and visual blurring. Ophthalmology review confirmed papilloedema. Pre-operative brain MRI image demonstrated a hyperintense lesion in the third ventricle, consistent with a colloid cyst [
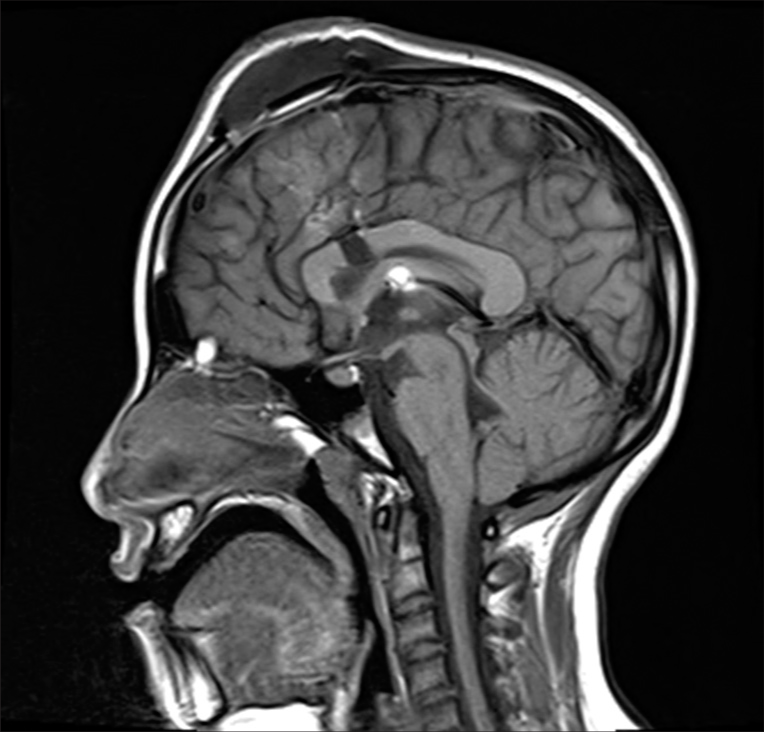
Postoperative brain magnetic resonance imaging (MRI)/ computed tomography (CT) were done under sedation and MRI showed evidence of an 8 mm incision in the anterior part of the body of the corpus callosum [
She received inpatient dexamethasone 4 mg IV injections every 6 h for a total of 2 days followed by injections every 8 h. Aspiration of the CSF scalp collection was done with removal of 20 mL of clear xanthochromic CSF. Her behavioral symptoms then steadily improved and disappeared after 2 weeks from initiation of therapy. She was discharged on dexamethasone 2 mg PO b. d. for 5 days, followed by dexamethasone 1 mg PO b. d. for another 5 days. Her short-term memory has been slower to improve (as per her parents, there has been “95% improvement in memory”) and residual memory deficits are still present 6 months after the surgery, such as trouble remembering where she placed objects or memorizing word passages. According to her parents, she does not remember her episode of behavioral disturbance or most of the events that occurred during her second admission to the hospital. However, she recalls that she “was not talking much because she was afraid that she would say something wrong.” Her diplopia has resolved completely. At school, she maintains good academic performance.
DISCUSSION AND CONCLUSION
Although the number of callosal fibers is determined at around birth, myelination and functional development of the corpus callosum continues into adolescence in normal children.[
There is a growing body of literature demonstrating the association between weakened integrity of the corpus callosum and behavioral abnormalities. For instance, children with AgCC have been shown to exhibit autistic-like behaviors in the realm of social interaction and communication, such as difficulty in initiating and sustaining conversation with patterns of speech that is “meaningless” or “out of place,” recognition of emotion as well as interpretation of humor and non-literal language expressions.[
Further studies demonstrating the role of callosal integrity in regulating behavior show that individuals with antisocial personality disorders have underlying structural callosal abnormalities, such as increased callosal white matter volume and length and increased interhemispheric connectivity, as well as a reduction in callosal thickness.[
In our present case, during the surgery for the third ventricular colloid cyst removal, anterior interhemispheric dissection was first done but was difficult and time consuming due to extensive thick arachnoid bands and gyral overlap. An 8 mm incision was then made in anterior part of the body of the corpus callosum to enter the right lateral ventricle. The colloid cyst was seen to be completely obstructing the foramen of Monro. The cyst was punctured and thick yellow granular fluid was released into the suction tip. Due to the complex thick attachments, the wall of the cyst was dissected off the fornix and deep cerebral veins with great care. Eventually, the cyst delivered in-toto through the callosotomy. Postoperative brain MRI/CT showed no significant pathology, with only mild frontal parafalcine gyral edema worse on the right that can be expected to occur postoperatively.
Our patient has a number of unique features. Her memory deficits are not unusual given that the fornices might have been involved as the walls of the cyst were attached to and dissected from the fornix. However, severe neuropsychological impairment, and behavioral disinhibition in particular, is unusual after such limited callosotomy. In light of the corpus callosum’s role in behavior, it is possible that transection of the anterior callosal body fibers contributed to the features of behavioral disinhibition seen in our patient, especially given that she is at the critical age for callosal maturation and may be structurally and functionally vulnerable to surgical insult.
Another factor that might have potentially contributed to her behavioral symptoms, whether in isolation or in conjunction with disruption of callosal fibers, was postoperative edema involving her prefrontal cortex, which is responsible for maintaining social appropriateness among other complex cognitive functions such as reasoning, decision-making, and personality expression, as well as the anterior cingulate cortex, which is also involved in inhibition.[
To the best of the authors’ knowledge, this is the first report in literature to describe behavioral disinhibition occurring as a sequelae to a surgical procedure involving callosotomy. Our finding emphasizes the importance of taking into account behavioral complications when considering callosotomy and ensuring that risk for such a complication is conveyed as part of the informed consent for performing callosotomy.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
The completion of this case report would not have been possible without the guidance and participation of Dr. Abdulla Qassim. His contributions are sincerely appreciated and gratefully acknowledged.
References
1. Asadi-Pooya AA, Sharan A, Nei M, Sperling MR. Corpus callosotomy. Epilepsy Behav. 2008. 13: 271-8
2. Badaruddin DH, Andrews GL, Bolte S, Schilmoeller KJ, Schilmoeller G, Paul LK. Social and behavioral problems of children with agenesis of the corpus callosum. Child Psychiatry Hum Dev. 2007. 38: 287-302
3. Brown WS, Paul LK. The neuropsychological syndrome of agenesis of the corpus callosum. J Int Neuropsychol Soc. 2019. 25: 324-30
4. El-Baba RM, Schury M, editors. Neuroanatomy, frontal cortex. StatPearls. Treasure Island, FL: StatPearls Publishing; p. n.d. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554483 [Last accessed on 2023 Jan 06]
5. Galaburda AM, Rosen GD, Sherman GF. Individual variability in cortical organization: Its relationship to brain laterality and implications to function. Neuropsychologia. 1990. 28: 529-46
6. Goldstein A, Covington B, Mahabadi N, Mesfin F, editors. Neuroanatomy, corpus callosum. Statpearls. Treasure Island, FL: StatPearls Publishing; 2022. p. Available from: https://www.ncbi.nlm.nih.gov/books/NBK448209 [Last accessed on 2023 Jan 06 ]
7. Hinkley LB, Marco EJ, Findlay AM, Honma S, Jeremy RJ, Strominger Z. The role of corpus callosum development in functional connectivity and cognitive processing. PLoS One. 2012. 7: e39804
8. Itaya S, Ueda Y, Kobayashi Z, Tomimitsu H, Kobayashi D, Shintani S. Bilateral frontal lobe vasogenic edema resulting from hypertrophic pachymeningitis due to granulomatosis with polyangiitis. Intern Med. 2017. 56: 3353-5
9. Kim DS, Yang KH, Kim TG, Chang JH, Chang JW, Choi JU. The surgical effect of callosotomy in the treatment of intractable seizure. Yonsei Med J. 2004. 45: 233
10. Kolb B, Mychasiuk R, Muhammad A, Li Y, Frost DO, Gibb R. Experience and the developing prefrontal cortex. Proc Natl Acad Sci U S A. 2012. 109: 17186-93
11. Lassonde M, Sauerwein H, Chicoine AJ, Geoffroy G. Absence of disconnexion syndrome in callosal agenesis and early callosotomy: Brain reorganization or lack of structural specificity during ontogeny?. Neuropsychologia. 1991. 29: 481-95
12. Luders E, Thompson PM, Toga AW. The development of the corpus callosum in the healthy human brain. J Neurosci. 2010. 30: 10985-90
13. Nehring S, Tadi P, Tenny S, editors. Cerebral edema. StatPearls. Treasure Island, FL: StatPearls Publishing; 2022. p. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537272 [Last accessed on 2023 Jan 06 ]
14. Paul LK. Developmental malformation of the corpus callosum: A review of typical callosal development and examples of developmental disorders with callosal involvement. J Neurodev Disord. 2011. 3: 3-27
15. Raine A, Lencz T, Taylor K, Hellige JB, Bihrle S, Lacasse L. Corpus callosum abnormalities in psychopathic antisocial individuals. Arch Gen Psychiatry. 2003. 60: 1134-42
16. Rasgon N, Ananth J, Mena I, Krout B, Boone K. Agenesis of corpus callosum and dementia of the Alzheimer’s type: A review and case report. Can J Psychiatry. 1994. 39: 429-32
17. Roxanas MG, Massey JS, Chaganti J. Antisocial behaviour and lying: A neuropsychiatric presentation of agenesis of the corpus callosum. Australas Psychiatry. 2014. 22: 461-6
18. Symington SH, Paul LK, Symington MF, Ono M, Brown WS. Social cognition in individuals with agenesis of the corpus callosum. Soc Neurosci. 2010. 5: 296-308
19. Taylor M, David AS. Agenesis of the corpus callosum: A United Kingdom series of 56 cases. J Neurol Neurosurg Psychiatry. 1998. 64: 131-4
20. Uda T, Kunihiro N, Umaba R, Koh S, Kawashima T, Ikeda S. Surgical aspects of corpus callosotomy. Brain Sci. 2021. 11: 1608
21. Young PA, Young PH, Tolbert DL, editors. Basic Clinical Neuroscience. Netherlands: Wolters Kluwer; 2015. p.