- Department of Neurosurgery, Kumamoto City Hospital, Kumamoto, Japan
- Department of Neurosurgery, Kumamoto Red Cross Hospital, Kumamoto, Japan
- Department of Neurosurgery, Kumamoto University, Kumamoto, Japan
Correspondence Address:
Yushin Takemoto
Department of Neurosurgery, Kumamoto University, Kumamoto, Japan
DOI:10.4103/2152-7806.181979
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Takemoto Y, Matsumoto J, Ohta K, Hasegawa S, Miura M, Kuratsu J. Bilateral posterior fossa chronic subdural hematoma treated with craniectomy: Case report and review of the literature. Surg Neurol Int 06-May-2016;7:
How to cite this URL: Takemoto Y, Matsumoto J, Ohta K, Hasegawa S, Miura M, Kuratsu J. Bilateral posterior fossa chronic subdural hematoma treated with craniectomy: Case report and review of the literature. Surg Neurol Int 06-May-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/bilateral-posterior-fossa-chronic-subdural-hematoma-treated-with-craniectomy-case-report-and-review-of-the-literature/
Abstract
Background:Posterior chronic subdural hematomas (pCSHs) are rare. Their diagnosis and treatment are difficult.
Description:A 69-year-old woman was admitted to our hospital with nausea, headache, and mild consciousness disturbance. Computed tomography and magnetic resonance imaging showed bilateral pCSH. To prevent further neurological deterioration, we performed surgery under general anesthesia by midline suboccipital craniectomy. Unexpected bleeding from a developed circuitous occipital sinus was stopped with hemoclips. After hematoma removal, she recovered and was transferred to a rehabilitation hospital. By the 19th postoperative day, she had developed no neurologic deficits.
Conclusion:This experience demonstrates the risk of blind surgical therapy in patients with pCSH. In such patients, posterior fossa craniectomy may be preferable in terms of diagnosis and safe treatment.
Keywords: Chronic subdural hematoma, craniectomy, posterior fossa
INTRODUCTION
Most chronic subdural hematomas (CSHs) are supratentorial. Posterior fossa CSHs (pCSHs) are rare, their diagnosis is difficult, and their pathogenesis is not well understood.[
CASE REPORT
Presentation and clinical evaluation
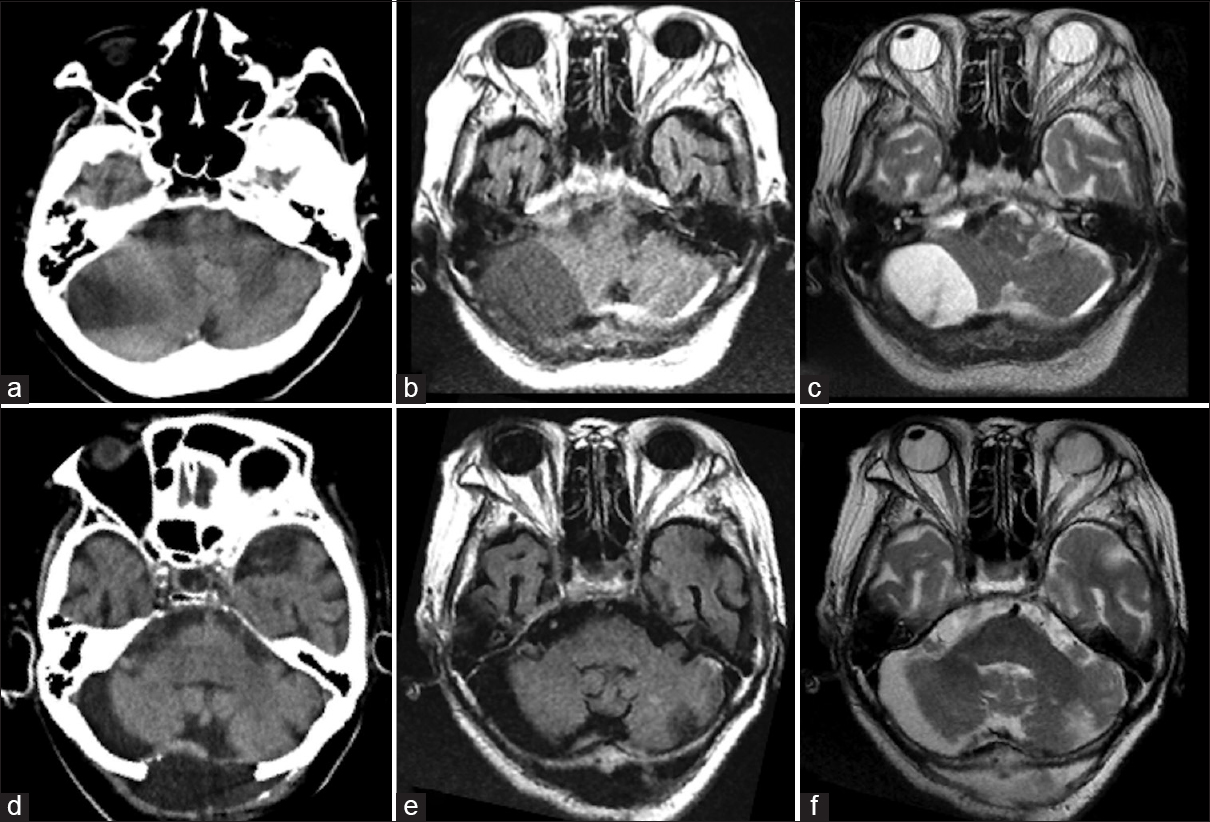
This 69-year-old woman suddenly developed nausea and headache followed on the next day by bouts of vomiting and gradual mild consciousness disturbance. At the time of admission to our hospital, she had mild consciousness disturbance (Glasgow Coma Scale [GCS] = 14) and her drowsiness increased (GCS = 13). She also had right dominant limb ataxia. She had undergone mitral valve replacement with a mechanical valve earlier and was on anticoagulation therapy. She also suffered from diabetes mellitus, rheumatoid arthritis, had an old vertebral compression fracture, old pulmonary tuberculosis, and an old brain infarct from cardiogenic embolism. There was no history of trauma. As laboratory data showed hypercoagulation (prothrombin time-international normalized ratio: 6.0), we reversed her status by administering Vitamin K. Computed tomography (CT) revealed a low- to high-density area in the subdural space of the posterior fossa and narrowing of the fourth ventricle [
Figure 1
Pre- and post-operative computed tomography and magnetic resonance imaging scans. (a) Preoperative computed tomography showed a low-density area in the right cerebellar hemisphere and a partly isodense area that was difficult to diagnose. (b and c) Preoperative magnetic resonance imaging (b, T1-weighted image; c, T2-weighted image) showing a subdural hematoma. The intensity is different on these images. (d) Postoperative computed tomography showing removal of the hematoma. (e and f) Postoperative magnetic resonance imaging showing removal of the hematoma (e, T1-weighted; f, T2-weighted)
Surgery and pathology
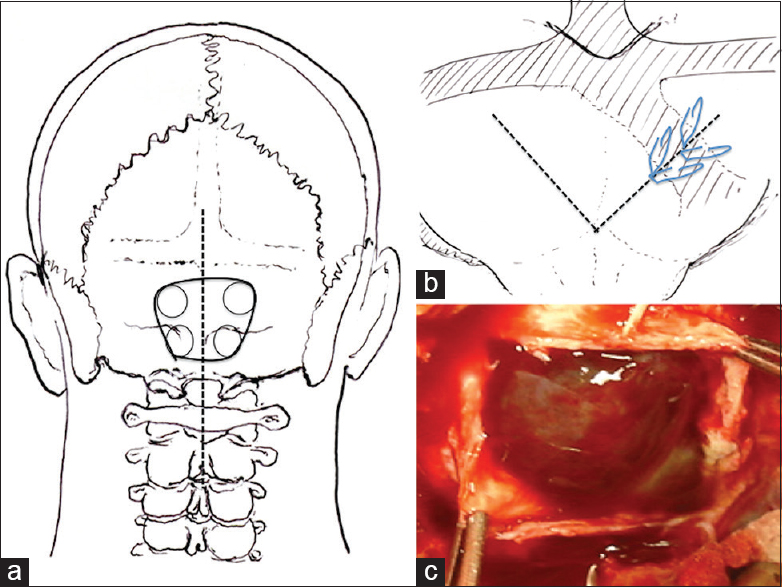
With the patient under general anesthesia and in the prone position, we used the midline suboccipital approach and performed bilateral suboccipital craniectomy [
Figure 2
Operative procedure. (a) A straight skin incision (dotted line) was made for midline bilateral suboccipital craniectomy (black solid line). (b) A V-shaped dural incision was made (dotted line) and the winding occipital sinus was clipped with hemoclips (slanted line). (c) After dural incision, the outer membrane of the chronic subdural hematoma was observed
Postoperative course
Immediately after surgery, her dizziness and consciousness disturbance disappeared. The drain was removed on the postoperative day (POD) 1. CT and MRI studies showed no residual mass effect in the fourth ventricle [Figure
DISCUSSION
As pCSH is uncommon, especially in adults, its natural history, pathogenesis, and effective treatment remain to be fully elucidated. The anatomic characteristics of posterior fossa- and supratentorial CSH (sCSH) are different; vascular injuries are rare.[
The diagnosis of pCSH is difficult; this may account for the low number of reported cases. Before the introduction of CT, most subdural hematomas in the posterior fossa were diagnosed intraoperatively or upon autopsy.[
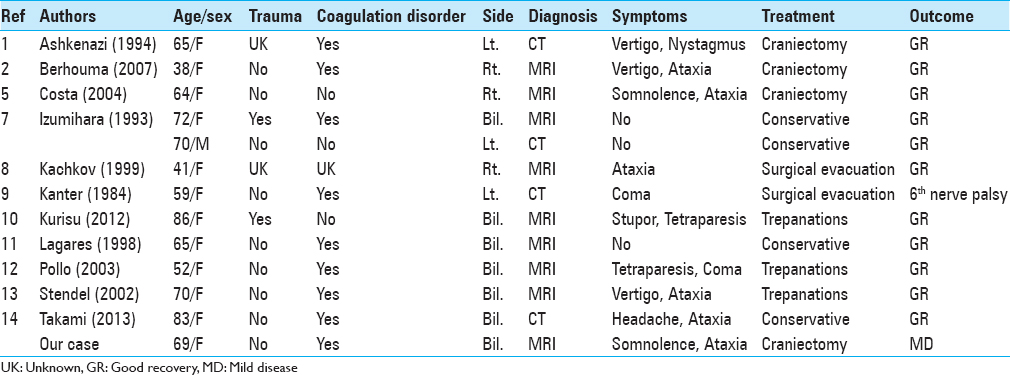
Izumihara et al.[
Among 4 conservatively treated patients with good outcomes, 3 manifested no posterior fossa symptoms.[
The mortality rate of CSH patients treated by craniectomy and burr hole craniostomy is not significantly different although craniectomy tends to elicit more perioperative complications than burr hole craniostomy. Therefore, burr hole (or twist-drill) craniostomy and placement of a closed drainage system are now the first choice surgical treatment due to its low morbidity and mortality rate.[
Nonetheless, the surgical treatment of patients with pCSH must be considered carefully. Dural sinuses such as the occipital-, sigmoid-, and transverse sinus and some relatively large emissary veins run in different courses on the surface layer of the posterior fossa. Their evaluation is difficult and not many patients with CSH are subjected to preoperative cerebral angiography. Consequently, intraoperative bleeding may occur. In fact, we encountered unexpected intraoperative bleeding from a circuitous occipital sinus. This sinus flows into the left or right side of the confluence of a sinus or transverse sinus, while most of these sinuses are single, missing, or double sinuses can exist. The occipital sinus is well developed in the perinatal period, and in most cases, it undergoes gradual involution; however, a well-developed occipital sinus may persist into adulthood.[
When intraoperative bleeding from the occipital sinus occurs, as it did in our patient, it may be difficult to control in patients subjected to burr hole surgery. To operate patients with pCSH, particularly those with bilateral lesions, we recommend general anesthesia and the prone position because the midline suboccipital approach is needed. However, prolonged general anesthesia increases the risk to the patient.[
As it is difficult to make a preoperative diagnosis of pCSH, the histologic study may help to reach a differential diagnosis although a relatively large operative field is required for the harvest of tissue specimens. To obtain satisfactory treatment outcomes, the location of the CSH, posterior fossa or supratentorial, must be known and the best treatment strategy, craniectomy or burr hole craniostomy, must be carefully chosen. Based on our experience, we recommend craniectomy with a sufficient operative field in patients with pCSH who must be placed under general anesthesia and operated in the prone position. More case reports must be collected to identify the best treatment in patients with pCSH.
CONCLUSION
The diagnosis of pCSH is difficult as is choosing the appropriate therapeutic method. Our patient alerts to the danger of blind surgical therapy in patients with pCSH. To operate pCSH patients under general anesthesia and in the prone position, we suggest that craniectomy is a safe, effective surgical procedure in terms of diagnosis and safety of the treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
I would like to express my sincere gratitude to my supervisor, Professor Junichi Kuratsu for providing me this precious study opportunity. I especially would like to express my deepest appreciation to my supervisor, Dr. Jun Matsumoto for his elaborated guidance, considerable encouragement, and invaluable discussion that make my research of great achievement and my study life unforgettable.
References
1. Ashkenazi E, Pomeranz S. Nystagmus as the presentation of tentorial incisure subdural haematoma. J Neurol Neurosurg Psychiatry. 1994. 57: 830-1
2. Berhouma M, Houissa S, Jemel H, Khaldi M. Spontaneous chronic subdural hematoma of the posterior fossa. J Neuroradiol. 2007. 34: 213-5
3. Capistrant T, Goldberg R, Shibasaki H, Castle D. Posterior fossa subdural haematoma associated with anticoagulant therapy. J Neurol Neurosurg Psychiatry. 1971. 34: 82-5
4. Ciembroniewicz JE. Subdural hematoma of the posterior fossa. Review of the literature with addition of three cases. J Neurosurg. 1965. 22: 465-73
5. Costa LB, de Andrade A, Valadão GF. Chronic subdural hematoma of the posterior fossa associated with cerebellar hemorrhage: Report of rare disease with MRI findings. Arq Neuropsiquiatr. 2004. 62: 170-2
6. Das AC, Hasan M. The occipital sinus. J Neurosurg. 1970. 33: 307-11
7. Izumihara A, Orita T, Kajiwara K, Tsurutani T. Simultaneous supra- and infratentorial chronic subdural hematoma. Eur J Radiol. 1993. 16: 183-5
8. Kachkov IA, Rusinov AI, Stashuk GA. Chronic subdural hematoma of the posterior cranial fossa. Zh Vopr Neirokhir Im N N Burdenko. 1999. 1: 30-1
9. Kanter R, Kanter M, Kirsch W, Rosenberg G. Spontaneous posterior fossa subdural hematoma as a complication of anticoagulation. Neurosurgery. 1984. 15: 241-2
10. Kurisu K, Kawabori M, Niiya Y, Ohta Y, Mabuchi S, Houkin K. Bilateral chronic subdural hematomas of the posterior fossae. Neurol Med Chir (Tokyo). 2012. 52: 822-5
11. Lagares A, Domínguez J, Lobato RD, González P. Bilateral posterior fossa subdural haematomas secondary to anticoagulant therapy. Acta Neurochir (Wien). 1998. 140: 1097-8
12. Pollo C, Meuli R, Porchet F. Spontaneous bilateral subdural haematomas in the posterior cranial fossa revealed by MRI. Neuroradiology. 2003. 45: 550-2
13. Stendel R, Schulte T, Pietilä TA, Suess O, Brock M. Spontaneous bilateral chronic subdural haematoma of the posterior fossa. Case report and review of the literature. Acta Neurochir (Wien). 2002. 144: 497-500
14. Takami H, Oshiro N, Hiraoka F, Murao M, Ide T. Rapid resolution of a spontaneous large chronic subdural haematoma in the posterior fossa under conservative treatment with platelet administration to aplastic anaemia. Clin Neurol Neurosurg. 2013. 115: 2236-9
15. Weigel R, Krauss JK, Schmiedek P. Concepts of neurosurgical management of chronic subdural haematoma: Historical perspectives. Br J Neurosurg. 2004. 18: 8-18