- Department of Neurosurgery, Faculdade de Medicina de São José do Rio Preto, São José do Rio Preto, Brazil.
- Department of Neurosurgery, Universidade Federal de São Paulo, São Paulo, Brazil.
Correspondence Address:
Ricardo Lourenço Caramanti
Department of Neurosurgery, Faculdade de Medicina de São José do Rio Preto, São José do Rio Preto, Brazil.
DOI:10.25259/SNI_166_2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ricardo Lourenço Caramanti, Feres Eduardo Chaddad Neto, Lucas Crociati Meguins, Carlos Eduardo Rocha, Dionei Freitas de Moraes, Mário José Góes. Brain metastasis of Merkel cell carcinoma – A rare case report. 10-Sep-2019;10:172
How to cite this URL: Ricardo Lourenço Caramanti, Feres Eduardo Chaddad Neto, Lucas Crociati Meguins, Carlos Eduardo Rocha, Dionei Freitas de Moraes, Mário José Góes. Brain metastasis of Merkel cell carcinoma – A rare case report. 10-Sep-2019;10:172. Available from: http://surgicalneurologyint.com/surgicalint-articles/9618/
Abstract
Background: Merkel cell carcinoma (MCC) is a rare neuroendocrine skin tumor. In our knowledge, only 30 cases of brain metastasis were reported in literature. The authors report a case of 57-year-old male with elevated intracranial pressure signs, which a frontal mass with pathological diagnosis of MCC.
Case Description: A 57-year-old male was admitted with a 3-month history of progressive headache, associated with nausea and dizziness. The magnetic resonance imaging showed a left frontal lobe, parasagittal, and nodular lesion with perilesional edema. The patient underwent complete surgical resection with success. The adjuvant treatment was radiotherapy and chemotherapy.
Conclusion: In our knowledge, there is a little number of cases of MCC reported in literature. Surgical management is considered in cases with intracranial hypertension or focal signs. The adjuvant treatment options are immunotherapy and radiotherapy.
Keywords: Brain metastasis, Merkel cell carcinoma, Skin neoplasm metastasis
INTRODUCTION
Merkel cell carcinoma (MCC) is a rare neuroendocrine tumor of the skin. The incidence is from 0.15 to 0.79 cases per 100,000 habitants which present local infiltration of lymph nodes and distant metastasis in 50% of the cases.[
The most common neurological symptoms are linked with elevated intracranial pressure. Brain magnetic resonance imaging (MRI) is the best imaging exam, despite it has unspecific findings.
There are no official treatment guidelines available; however, consensus showing better outcomes in patients underwent a brain metastasis resection exists.[
The aim of this study is to describe a rare case of MCC without primary skin lesion found.
CASE REPORT
A 56-year-old male, Caucasian, with 3-month history of progressive bilateral frontal headache associated with weight loss and dizziness, presented clinical worsening with nausea and confusion periods 2 days before hospitalization.
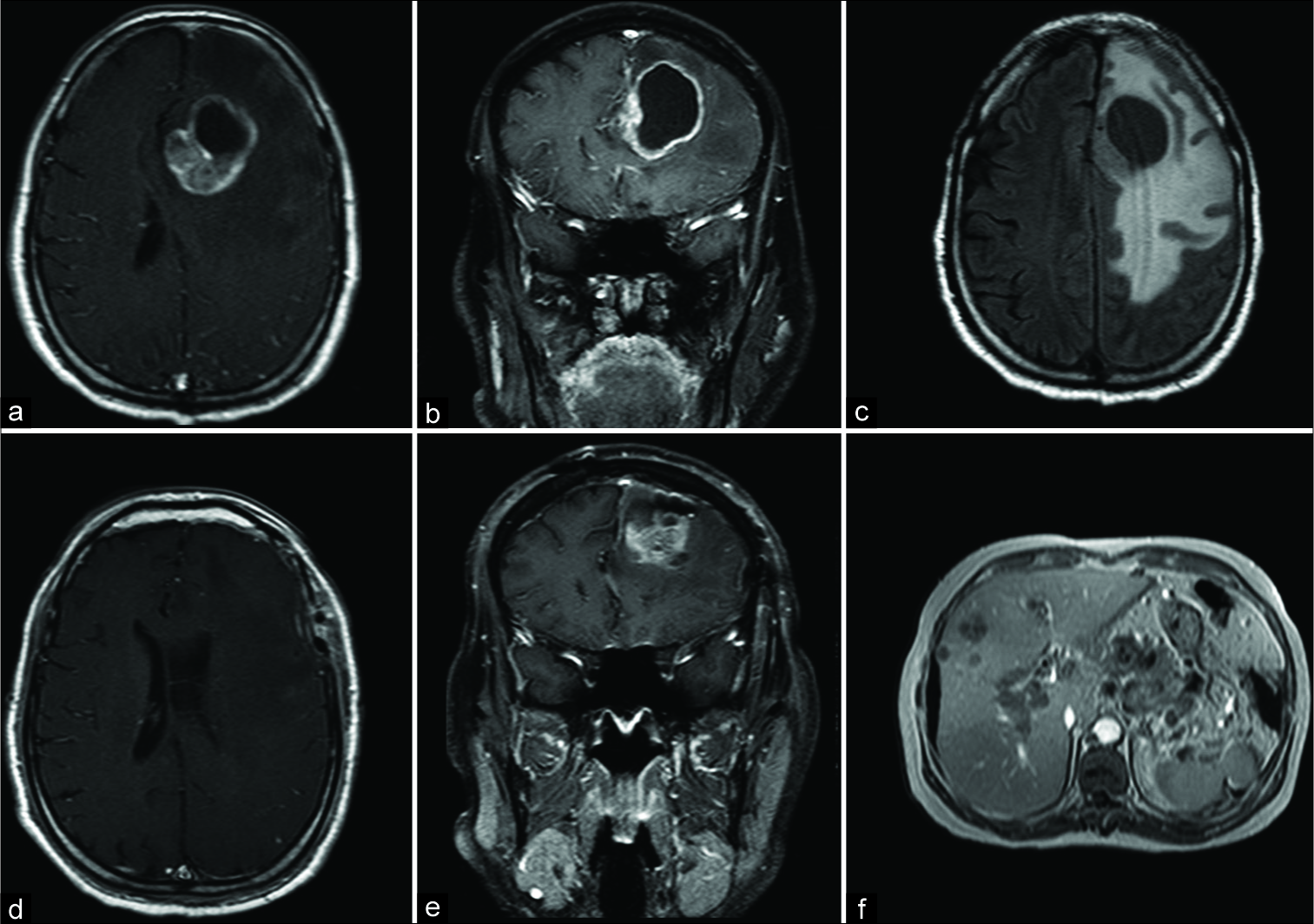
On admission, the patient presented without pupillary changes or focal signs. The basic biochemical tests were normal, and the MRI showed a cystic/nodular mass effect lesion, with important perilesional edema in the left frontal lobe, with approximately 5 cm, isointense in T1 and T2 sequences. The lesion presented contrast enhancement and restriction of nodular part in the diffusion sense. On spectroscopy, there was high choline/creatine and choline/N– Acetylaspartate ratios suggesting a brain metastasis or glioblastoma [
Figure 1:
(a and b) Magnetic resonance imaging (MRI) in axial and coronal gadolinium showing a solid cystic paramedian mass effect lesion with nodular and wall contrast enhancement. (c) MRI with axial flair sequence which an important frontal and parietal brain edema. (d and e) Postoperative axial and coronal gadolinium MRI showing complete tumor resection. (f) Abdomen MRI with multiple liver hypointense metastatic lesions.
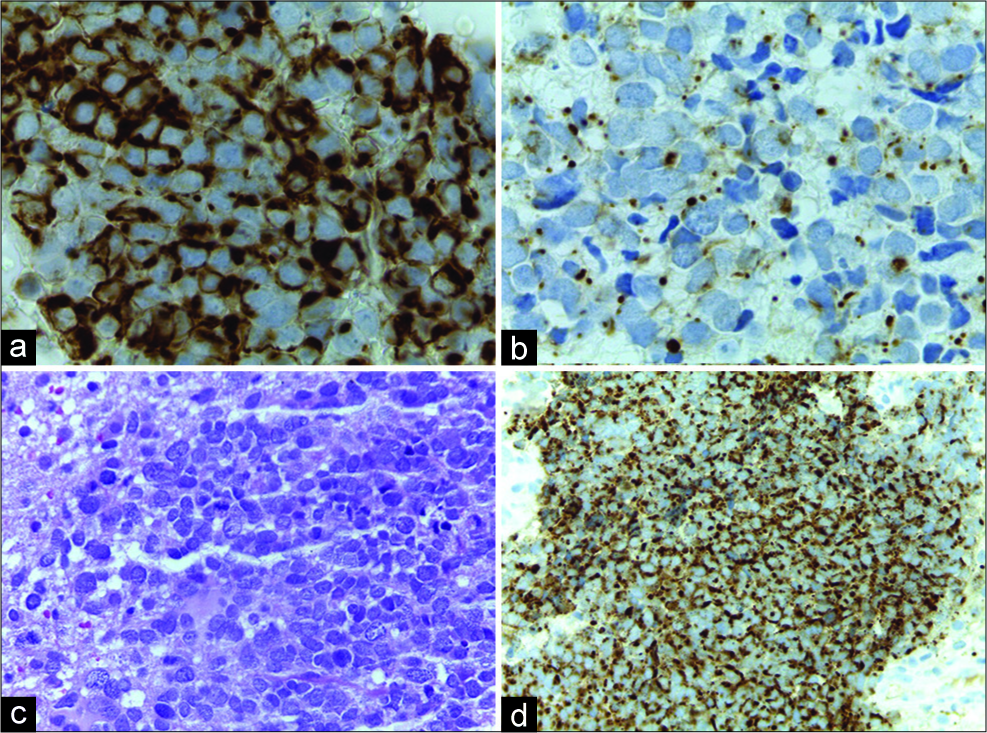
Due to the important mass effect and size, we opted for the surgical treatment with complete microsurgical resection. The patient evolved with complete neurological improvement, and the biopsy confirms MCC metastasis showing CK20, chromogranin, CD56 positive expressions, and with ki67 of 30% [
A screening to search for other sites of metastases was realized showing liver nodules in CT of the abdomen. The complete skin physical examination was performed without suspect lesions primary lesions.
The patient was forwarded to adjuvant treatment using radiotherapy with 50 Gy and chemotherapy. He evolved to death 3 months later due to hepatic complications.
DISCUSSION
MCC is a rare neuroendocrine tumor characterized by small cells with round nuclei, monomorphic, with basophilic nucleus, and cytoplasm minimum associated with high mitosis number and apoptotic bodies.[
The immunohistochemical is critical to differentiation of the MCC, showing cytokeratins 8, 18, and 20 positives, with sensitivity >90%. The cytokeratin CK20 is mainly used to differentiate lung small-cell carcinoma and was positive in 87% of MCC. The A - chromogranine is present in 52% and enolase in 50% of cases. We can still find somatostatin, neurofilament, CD 56 and synaptophysin positives.[
Despite its rarity, Hodgson et al. showed in their study that there was an increase in the number of cases in the past decades, from 0.15 to 0.79 cases per 100,000 inhabitants.[
It typically affects elderly Caucasians with light skin types with a mean age of 69 years, however, can occur in immunosuppressed young people including: organ transplant recipients, HIV-infected individuals, and those with B-cell malignancies, with local recurrence tendencies.
Besides the immunodeficiency, other factors such as Merkel cell polyomavirus, ultraviolet radiation exposure appears to contribute to tumor genesis from immature totipotential stem cells.[
The skin lesion can present rapidly growing, painless, firm, nontender, shiny, flesh-colored or bluish-red, intracutaneous nodule, commonly located in head and neck (29%), upper limbs (24%), lower limbs (21%), stem (8%), and rarely vulva (5%), however, in 4% of cases, it not be found.[
The Memorial Sloan Kettering Cancer Center in 1999 proposed a size-based grading system and correlated it with survival: Stage I – primary tumor <2 cm; Stage II –primary tumor >2 cm; Stage III – regional disease, and Stage IV – distant disease (metastasis). The survival rates for each stage are for Stage I – 81%, Stage II – 67%, Stage III – 52%, and Stage IV – 11% in 5 years.[
Extracranial metastasis is common in liver, lung, and the skin itself. Intracranial metastasis is uncommon, presenting a little number of cases published in the literature. Feletti et al. reviewed 15 cases showing that the most common sites of metastasis are parietal lobe, cerebellum, and meninges with 3 cases reported for each of these regions. The intracranial involvement can be directly or by the contiguity in case of head lesions.[
Most common symptoms are the elevation of intracranial pressure, focal signs such as headache, dizziness, vomiting, visual alterations, paresis, parenthesis, and mental confusion.[
MRI is the best image method to detect the MCC brain metastasis; however, it shows unspecific findings such as hypo/isointense signal in T1 and T2 sequences, with homogeneous enhancing postgadolinium administration. The surrounding edema can be extensive, presenting a bright signal in T2 and flair sequences.[
The main differential diagnoses of MCC are glioblastoma, brain abscess, and other metastatic tumors. In the case of brain abscess, the surgical findings and a simple histological analysis can differentiate it. Other tissue metastases and glioblastoma need immunohistochemical tests for diagnosis.[
There is no official treatment guideline, but according to Harary et al., resection rate is an independent prognostic factor causing benefits such as control of intracranial hypertension and improvement of focal signs caused by mass effect. Other important prognostic factors are burden of disease, age, and adjuvant therapy.[
The adjuvant treatment options are mainly a role brain radiotherapy with approximately 50 Gy and cytotoxic chemotherapy, but the responses can be nondurable.[
Actually the immunotherapy with antibodies targeting the programmed cell death protein 1/programmed cell death ligand 1 such as avelumab, pembrolizumab, and nivolumab is an effective option, which is more commonly used in Stage III of disease.[
In our case, despite complete surgical resection, the patient had a poor prognosis, because he had an advanced disease stage (Stage IV) and a bad response to chemotherapy. We did not use immunotherapy because it was not available in public health system during your assistance.
CONCLUSION
MCC metastasis shows unspecific clinical and radiological findings. It needs to be suspected if the patient has previous compatible skin lesions or history of skin primary MCC. The diagnosis confirmed by immunohistochemical positivity to CK20 and A - chromogranine.
The gross total resection seems to provide a survival benefit and should be attempted always as possible if patient clinical condition permits.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abul-Kasim K, Söderström K, Hallsten L. Extensive central nervous system involvement in Merkel cell carcinoma: A case report and review of the literature. J Med Case Rep. 2011. 5: 35-
2. Allen PJ, Bowne WB, Jaques DP, Brennan MF, Busam K, Coit DG. Merkel cell carcinoma: Prognosis and treatment of patients from a single institution. J Clin Oncol. 2005. 23: 2300-9
3. Bichakjian CK, Lowe L, Lao CD, Sandler HM, Bradford CR, Johnson TM. Merkel cell carcinoma: Critical review with guidelines for multidisciplinary management. Cancer. 2007. 110: 1-2
4. Coggshall K, Tello TL, North JP, Yu SS. Merkel cell carcinoma: An update and review: Pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018. 78: 433-42
5. Feletti A, Marton E, Rossi S, Canal F, Longatti P, Billeci D. Pituitary metastasis of Merkel cell carcinoma. J Neurooncol. 2010. 97: 295-9
6. Fitzgerald TL, Dennis S, Kachare SD, Vohra NA, Wong JH, Zervos EE. Dramatic increase in the incidence and mortality from Merkel cell carcinoma in the United States. Am Surg. 2015. 81: 802-6
7. Gillenwater AM, Hessel AC, Morrison WH, Burgess M, Silva EG, Roberts D. Merkel cell carcinoma of the head and neck: Effect of surgical excision and radiation on recurrence and survival. Arch Otolaryngol Head Neck Surg. 2001. 127: 149-54
8. Harary M, Kavouridis VK, Thakuria M, Smith TR. Predictors of survival in neurometastatic Merkel cell carcinoma. Eur J Cancer. 2018. 101: 152-9
9. Heath M, Jaimes N, Lemos B, Mostaghimi A, Wang LC, Peñas PF. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: The AEIOU features. J Am Acad Dermatol. 2008. 58: 375-81
10. Heymann WR. Merkel cell carcinoma: Insights into pathogenesis. J Am Acad Dermatol. 2008. 59: 503-4
11. Hodgson NC. Merkel cell carcinoma: Changing incidence trends. J Surg Oncol. 2005. 89: 1-4
12. Honeybul S. Cerebral metastases from Merkel cell carcinoma: Long-term survival. J Surg Case Rep. 2016. 2016: rjw165-
13. Jabbour J, Cumming R, Scolyer RA, Hruby G, Thompson JF, Lee S. Merkel cell carcinoma: Assessing the effect of wide local excision, lymphonode dissection, and radiotherapy on recurrence and survival in early stage disease results from a review of 82 consecutive cases diagnosed between 1992 and 2004. Ann Surg Oncol. 2007. 14: 1943-52
14. Jagannathan J, Yen CP, Ray DK, Schlesinger D, Oskouian RJ, Pouratian N. Gamma knife radiosurgery to the surgical cavity following resection of brain metastases. J Neurosurg. 2009. 111: 431-8
15. Kalkanis SN, Kondziolka D, Gaspar LE, Burri SH, Asher AL, Cobbs CS. The role of surgical resection in the management of newly diagnosed brain metastases: A systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010. 96: 33-43
16. Kocher M, Soffietti R, Abacioglu U, Villà S, Fauchon F, Baumert BG. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952-26001 study. J Clin Oncol. 2011. 29: 134-41
17. Lien MH, Baldwin BT, Thareja SK, Fenske NA. Merkel cell carcinoma: Clinical characteristics, markers, staging and treatment. J Drugs Dermatol. 2010. 9: 779-84
18. Medina-Franco H, Urist MM, Fiveash J, Heslin MJ, Bland KI, Beenken SW. Multimodality treatment of Merkel cell carcinoma: Case series and literature review of 1024 cases. Ann Surg Oncol. 2001. 8: 204-8
19. Nabros L, Portnow J, Ammirati M, Baehring J, Brem H, Butowski N.editors. NCCN Clinical Practice Guidelines in Oncology for Central Nervous System Cancers (Version 1.2017). National Comprehensive Cancer Network. 2017. p.
20. Ott MJ, Tanabe KK, Gadd MA, Stark P, Smith BL, Finkelstein DM. Multimodality management of Merkel cell carcinoma. Arch Surg. 1999. 134: 388-92
21. Schadendorf D, Lebbé C, Zur Hausen A, Avril MF, Hariharan S, Bharmal M. Merkel cell carcinoma: Epidemiology, prognosis, therapy and unmet medical needs. Eur J Cancer. 2017. 71: 53-69
22. Stang A, Becker JC, Nghiem P, Ferlay J. The association between geographic location and incidence of Merkel cell carcinoma in comparison to melanoma: An international assessment. Eur J Cancer. 2018. 94: 47-60
23. Wick MR, Goellner JR, Scheithauer BW, Thomas JR, Sanchez NP, Schroeter AL. Primary neuroendocrine carcinomas of the skin (Merkel cell tumors). A clinical, histologic, and ultrastructural study of thirteen cases. Am J Clin Pathol. 1983. 79: 6-13
24. Zhao M, Meng MB. Merkel cell carcinoma with lymph node metastasis in the absence of a primary site: Case report and literature review. Oncol Lett. 2012. 4: 1329-34