- Department of Neurosurgery, Osaka City University Graduate School of Medicine, 1-4-3 Asahimachi, Abenoku,
- Department of Neurosurgery, Yao Tokushukai General Hospital, 1-11 Wakakusa-Cho, Yao, Osaka, Japan,
- Department of Neurosurgery, Al-Azhar University Faculty of Medicine-Nasr city, Cairo, Egypt.
Correspondence Address:
Tsutomu Ichinose
Department of Neurosurgery, Osaka City University Graduate School of Medicine, 1-4-3 Asahimachi, Abenoku,
DOI:10.25259/SNI_550_2019
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hiroshi Uda, Alhusain Nagm, Tsutomu Ichinose, Yohei Onishi, Masaki Yoshimura, Takashi Tsuruno, Kenji Ohata. Burr hole drainage without irrigation for chronic subdural hematoma. 02-May-2020;11:89
How to cite this URL: Hiroshi Uda, Alhusain Nagm, Tsutomu Ichinose, Yohei Onishi, Masaki Yoshimura, Takashi Tsuruno, Kenji Ohata. Burr hole drainage without irrigation for chronic subdural hematoma. 02-May-2020;11:89. Available from: https://surgicalneurologyint.com/surgicalint-articles/9991/
Abstract
Background: Chronic subdural hematoma (CSDH) is one of the most common neurosurgical conditions, with different strategies for treatment. Most recent trials favor the use of drainage to reduce the recurrence rate. However, few reports have discussed the efficacy of burr hole drainage without irrigation for treating CSDH. This study aimed to examine the efficacy of burr hole drainage without irrigation in a series of 385 symptomatic CSDH lesions.
Methods: This retrospective study included a series of 385 symptomatic CSDH lesions in 309 patients, who underwent burr hole drainage without irrigation, between September 2009 and August 2017 at the Department of Neurosurgery, Yao Tokushukai General Hospital, Japan. The risk of recurrence was evaluated based on the patients’ age, sex, preoperative magnetic resonance imaging (MRI) findings, preoperative anticoagulants, hematoma drainage rate, and bilaterality.
Results: Of the 385 lesions, 41 cases (16 with inadequate follow-up periods and 25 with contraindications for MRI) were excluded from the analysis. The overall recurrence rate in the index study was 4.9% (17/344 lesions). The effects of the preoperative hematoma volume and nonhyperintensity on T1-weighted imaging on the recurrence rate were significant.
Conclusion: Our findings indicated that burr hole drainage without irrigation is a good surgical modality in patients with CSDH, and preoperative MRI findings can evaluate the risk of recurrence.
Keywords: Burr hole, Chronic subdural hematoma, Drainage without irrigation, Magnetic resonance imaging, Risk factors
INTRODUCTION
Neurosurgeons often treat patients with chronic subdural hematoma (CSDH). Although CSDH is commonly thought to be a benign disease, the overall recurrence rate for CSDH is 13.1% in Japan, and the functional outcome at discharge is poor at 28.4%.[
Burr hole surgery is the most commonly employed procedure for the surgical treatment of CSDH.[
Few reports have described the efficacy of burr hole drainage without irrigation.[
Our surgical algorithm involves preoperative examination, burr hole drainage without irrigation, and postoperative management. This study aimed to examine the efficacy of burr hole drainage without irrigation in a series of 385 symptomatic CSDH lesions.
MATERIALS AND METHODS
Patient population
A series of 385 symptomatic CSDH lesions in 309 patients, who underwent burr hole drainage without irrigation, between September 2009 and August 2017 at the Department of Neurosurgery, Yao Tokushukai General Hospital, were included in this study. The participants included patients with ipsilateral lesions who underwent repeat surgery. None of the patients exhibited growth of the contralateral CSDH within 60 days after unilateral surgery. The patients consisted of 213 men and 96 women, with a mean age of 76.0 years (range, 33–106 years). Two hundred and forty-one patients had unilateral and 68 patients had bilateral subdural hematomas. Patients who were contraindicated for MRI or underwent ≤60 days of follow-up were excluded from this study. Those who underwent repeat surgery due to signs and symptoms of hematoma growth were defined as patients with recurrent lesions.
Imaging evaluation
The presence or absence of CSDH was determined with the aid of brain computed tomography (CT) before detailed brain MRI evaluation. The hematoma volume was measured on fluid-attenuated inversion recovery coronal images using a workstation (ZioStation®; Ziosoft, Osaka, Japan). The presence or absence of a septa within the hematoma cavity was evaluated on the basis of the presence or absence of black bands on T2*-weighted images.[
Surgical procedure
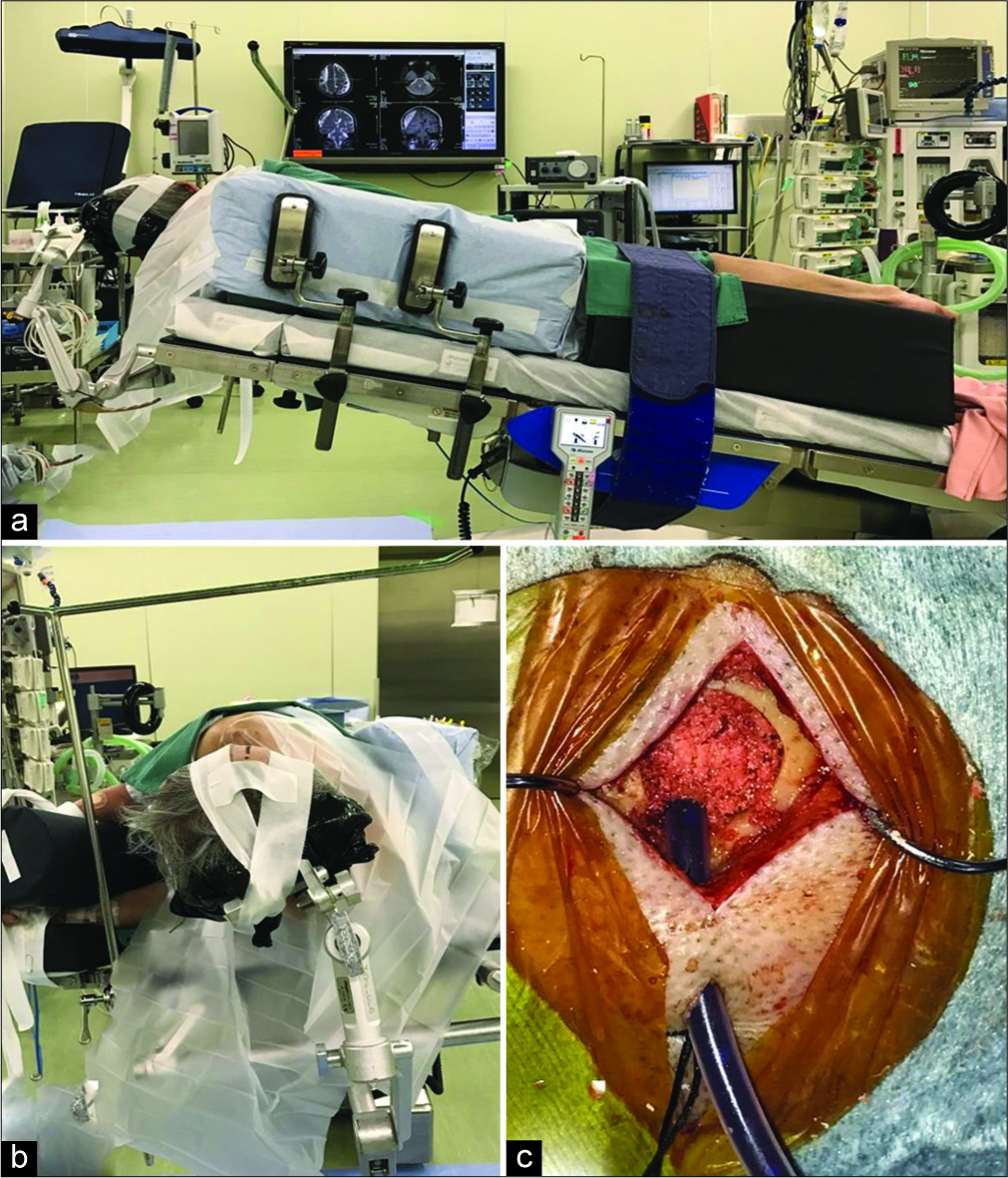
The patient was placed in the lateral position with the head fixed on a horseshoe-shaped pillow, provided that the burr hole site was at the highest point [
Figure 1:
Surgical procedure for burr hole drainage without irrigation. (a) The patient is placed in the lateral position, (b) the head is fixed within the head holder, and the burr hole is positioned at the highest point of the operative field, (c) the drainage tube is inserted at a depth of 3 cm into the hematoma cavity, and the burr hole is filled with bone dust and a cellulose sponge.
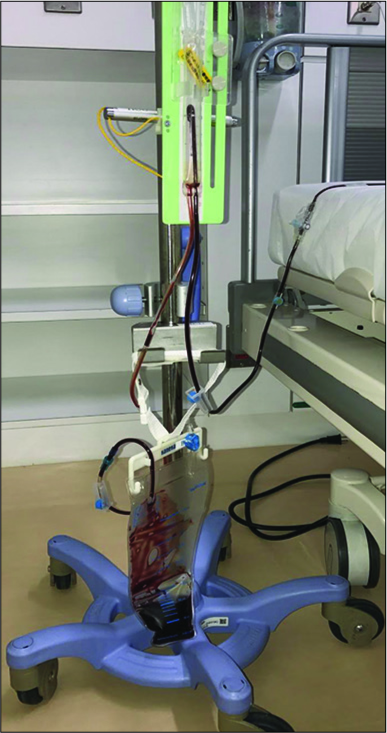
Postoperative drainage was performed in the supine position without head elevation. Slow drainage was performed with a semi-closed drainage circuit, set at the level of the external auditory canal [
Postoperative evaluation
Postoperative follow-up was performed by evaluation of the neurological status and brain CT. Oral administration was continued in cases of hematoma regrowth on the CT image, even if no worsening of neurological symptoms was observed. Repeat surgery was performed if any neurological symptoms resulting from hematoma regrowth were observed.
Statistical analysis
The risk factors for recurrence (i.e., age, sex, preoperative MRI findings, the presence or absence of preoperative antithrombotic therapy, hematoma drainage rate, and hematoma bilaterality) were evaluated using the Chi-squared test for sex, nonhyperintense lesions on T1WI, the presence or absence of septa, the presence or absence of preoperative antithrombotic therapy, hematoma drainage rate of <50%, and hematoma bilaterality, or the Mann–Whitney U-test for age and preoperative hematoma volume using SPSS (SPSS inc., Chicago, IL, USA).
RESULTS
Of the 385 lesions, 41 cases (16 with inadequate follow-up periods and 25 with contraindications for MRI) were excluded from the analysis. Seventeen (4.9%) out of 344 lesions exhibited recurrence. The mean time for recurrence was 26.4 days (range, 1–59 days). Perioperative complications consisted of subdural empyema (n = 3), cerebral contusion due to the drainage tube (n = 1), and cardiogenic cerebral embolism (n = 1). Subdural empyema was treated with drainage and antimicrobial therapy. Cerebral contusion without neurological symptoms improved with conservative treatment. Cardiogenic cerebral embolism improved after mechanical thrombectomy.
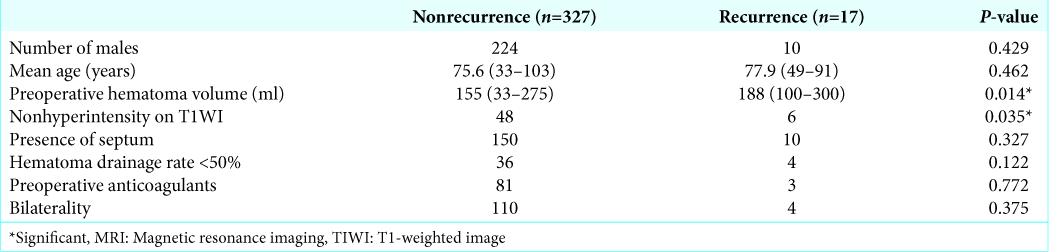
There were no significant differences in the recurrence rate between the male group (10/234 lesions) and the female group (7/110 lesions) (P = 0.429), the presence of septum group (10/160 lesions) and the absence of septum group (7/284 lesions) (P = 0.327), the hematoma drainage rate <50% group (4/40 lesions) and the hematoma drainage rate ≤50% group (13/304 lesions) (P = 0.122), or the presence of preoperative anticoagulants therapy group (3/84 lesions) and the absence of preoperative anticoagulants therapy group (14/260 lesions) (P = 0.772), according to the Chi-squared test.
On the other hand, there was a significant difference in the recurrence rate between nonhyperintensity on T1WI group (6/54 lesions) and hyperintensity on T1WI group (11/290 lesions) (P = 0.035), according to the Chi-squared test. There was no significant effect of age (nonrecurrence group: mean age 75.6 years [30–103 years] and recurrence group: mean age 77.9 years [49–91 years]) on the recurrence rate (P = 0.462), according to the Mann–Whitney U-test.
Our results indicated a significant effect of preoperative hematoma volume on the recurrence rate (P = 0.014), according to the Mann–Whitney U-test. The mean preoperative hematoma volume was 155 mL (33–275 mL) and 188 mL (100–300 mL) in the nonrecurrence and recurrence groups, respectively. Evaluation using the Youden index[
Figure 3:
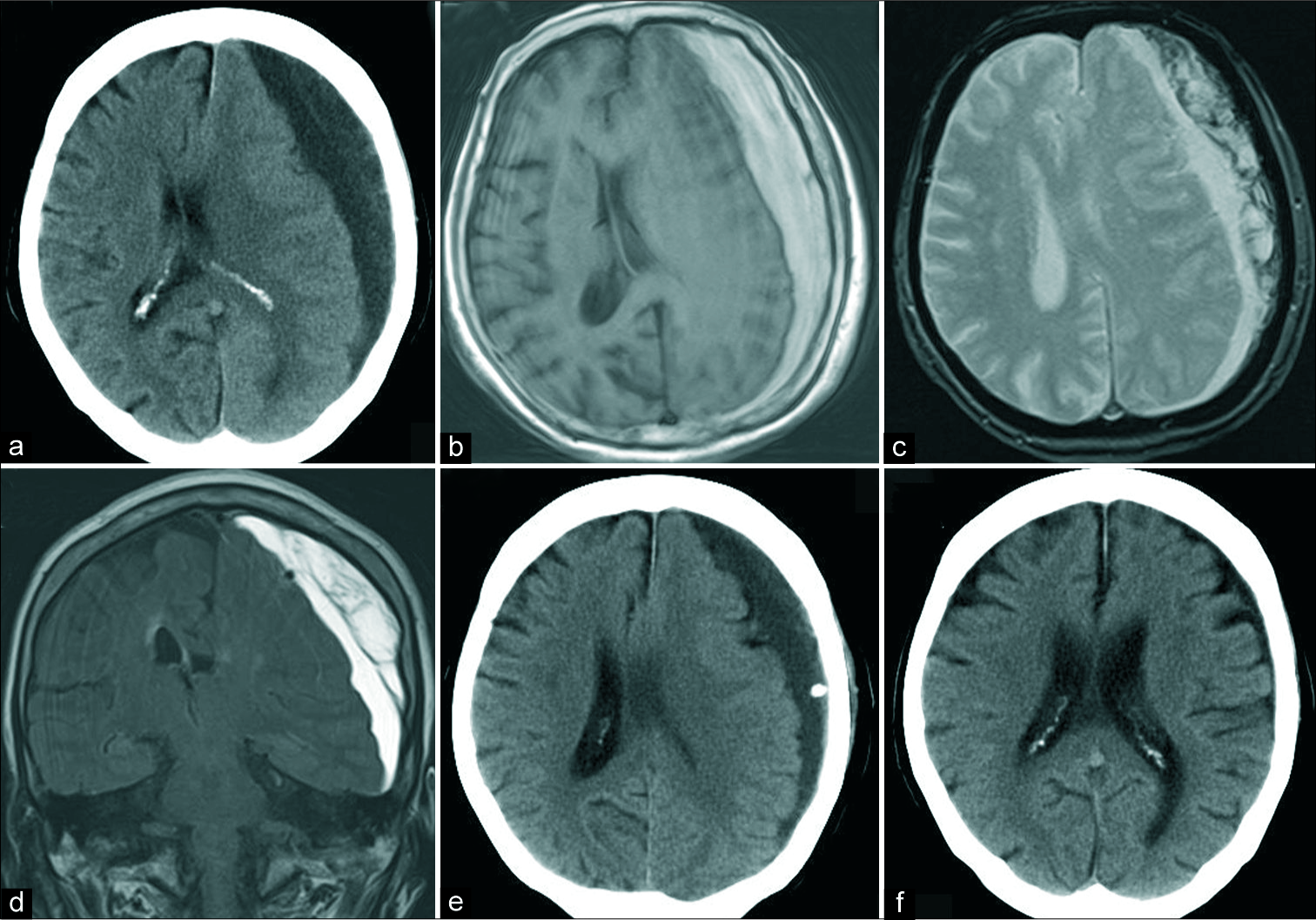
A 74-year-old woman with the left CSDH (a) the preoperative CT scan reveals a mixed density subdural hematoma with a midline shift, (b) hematoma showing hyperintensity on TIWI, (c) the presence of black bands on T2*-weighted images implies the presence of septa within the hematoma cavity, (d) the preoperative hematoma volume was 173 mL, as measured on fluid-attenuated inversion recovery coronal images, (e) CT scan 1 day after surgery revealing little air collection in hematoma cavity. The drainage rate was 36%. (f) The subdural hematoma disappeared 2 months after surgery. The patient recovered completely from right-sided hemiparesis in this case. CT: Computed tomography, CSDH: Chronic subdural hematoma.
Figure 4:
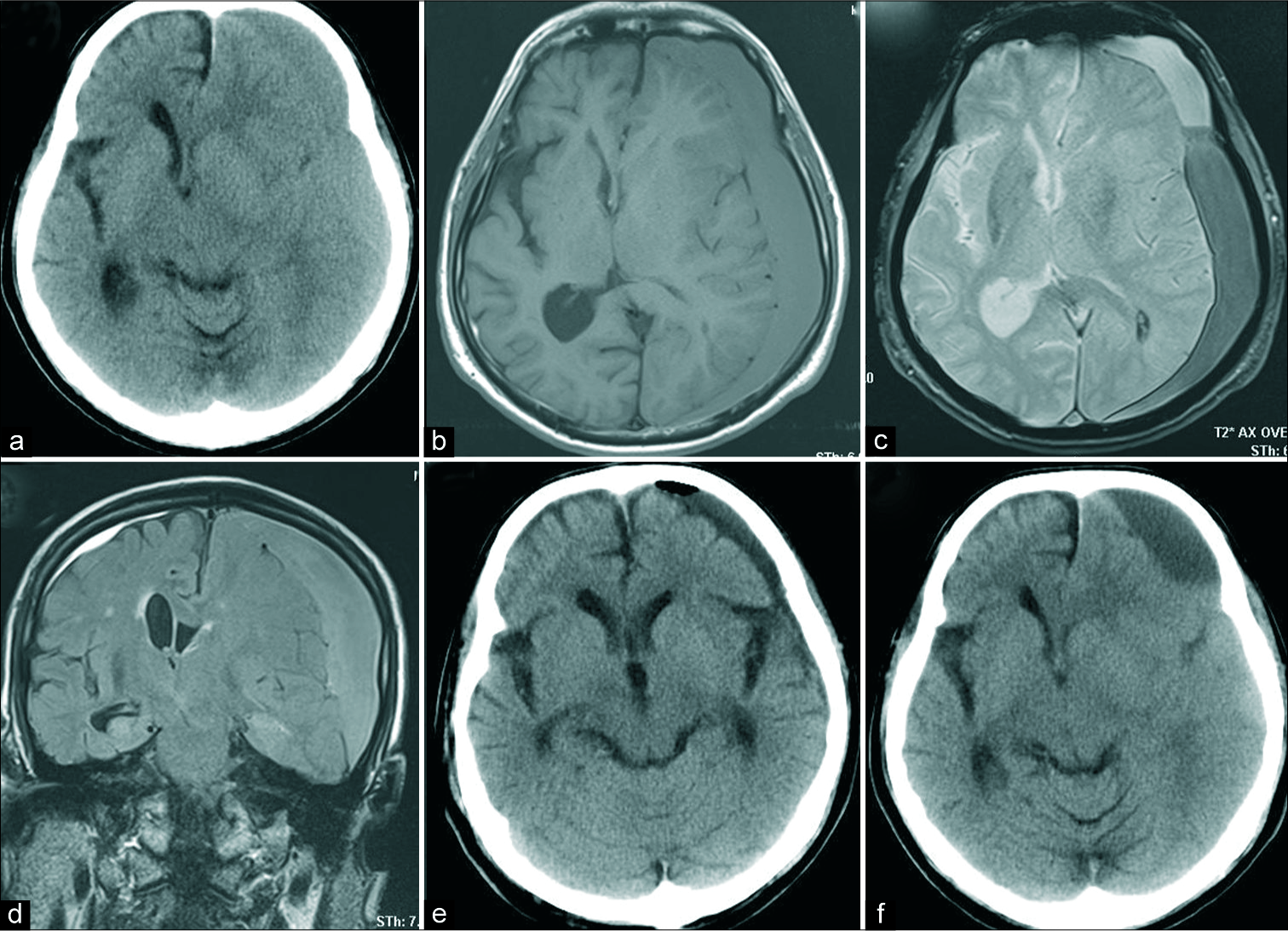
Recurrence in an 83-year-old man with bilateral CSDH (a) preoperative CT scans revealing midline shift with a hyperdense subdural hematoma, (b) hematoma showing low intensity on TIWI, (c) the presence of black bands on T2*-weighted images imply the presence of septa within the hematoma cavity, (d) the preoperative hematoma volume was 237 mL, as measured on fluid-attenuated inversion recovery coronal imaging, (e) CT scan revealing little air collection in the hematoma cavity, 1 day after surgery. The subdural hematoma drained very well. (f) The subdural hematoma recurred 23 days after surgery with right hemiparesis. CT: Computed tomography, CSDH: Chronic subdural hematoma.
This study indicated that burr hole drainage without irrigation is a good surgical option in patients with CSDH and that preoperative MRI findings can help evaluate the risk of recurrence.
DISCUSSION
Burr hole types for CSDH
It has been reported that CSDH has a relatively good prognosis, with a mortality rate of approximately 2%.[
Burr hole surgery is commonly performed to treat CSDH.[
Among the studies comparing burr hole drainage with irrigation to burr hole drainage without irrigation, some reported that surgery with irrigation reduces the recurrence rate,[
We should also evaluate the efficacy of Kampo medications for comparison with earlier reports. It has been reported that Kampo medication (e.g. goreisan and saireito) is effective in improving the symptoms of subdural hematoma,[
The surgeons at our index institute often place patients in the lateral position, such that the burr hole site is at the highest point. However, such positioning may increase the risk of decubitus and falls. Fortunately, appropriate sedation and gentle position fixation ensured no complications due to positioning at our institute. Sedation is induced by intravenous administration of midazolam (0.06–0.08 mg/kg body weight) under oxygen administration, and additional administration of midazolam (0.02–0.03 mg/kg body weight) depending on the sedation state. Moreover, pentazocine (15 mg) was administered intravenously to relieve pain. The dose was adjusted depending on age and the state of consciousness. There are no reports detailing the appropriate intraoperative positions in burr hole drainage without irrigation.
Three patients (0.8%) experienced perioperative complications, including subdural empyema, which was successfully treated in all patients. According to earlier reports, the incidence of subdural empyema after burr hole surgery is 0–2.1%.[
Nonhyperintensity on T1WI
This study identified nonhyperintensity on T1WI and a preoperative hematoma volume of ≥160 mL as risk factors for recurrence. Earlier studies have also reported nonhyperintensity on T1WI as a risk factor for recurrence.[
Adjuvant therapy for CSDH
Middle meningeal artery embolization has also been reported to be effective in the treatment of subdural hematomas that exhibit nonhyperintensity on T1WI.[
This was a single retrospective study. Future investigations should include a greater number of cases and prospective studies with larger samples.
CONCLUSION
We examined in detail, the efficacy of burr hole drainage without irrigation and the risk factors for recurrence of CSDH in a series of 385 symptomatic lesions. The overall recurrence rate was 4.9% and was the preoperative hematoma volume and nonhyperintensity on T1WI was identified as risk factors for recurrence. Burr hole drainage without irrigation is a good surgical option for CSDH cases, and preoperative MRI findings can help evaluate the risk of recurrence.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Ban SP, Hwang G, Byoun HS, Kim T, Lee SU, Bang JS. Middle meningeal artery embolization for chronic subdural hematoma. Radiology. 2018. 286: 992-9
2. Bradley WG. MR appearance of hemorrhage in the brain. Radiology. 1993. 189: 15-26
3. Brennan PM. The management and outcome for patients with chronic subdural hematoma: A prospective, multicenter, observational cohort study in the United Kingdom. J Neurosurg. 2017. 127: 732-9
4. Chen XU, Shiwen C, Lutao Y, Yao J. Burr-hole irrigation with closed-system drainage for the treatment of chronic subdural hematoma: A meta-analysis. Neurol Med Chir (Tokyo). 2016. 56: 62-8
5. Goto H, Ishikawa O, Nomura M, Tanaka K, Nomura S, Maeda K. Magnetic resonance imaging findings predict the recurrence of chronic subdural hematoma. Neurol Med Chir (Tokyo). 2015. 55: 173-8
6. Goto S, Kato K, Yamamoto T, Shimato S, Ohshima T, Nishizawa T. Effectiveness of goreisan in preventing recurrence of chronic subdural hematoma. Asian J Neurosurg. 2018. 13: 370-4
7. Gürbüz MS, Karaarslan N, Gök S, Soyalp C. Remote cerebellar haemorrhage after burr hole drainage of chronic subdural haematoma: A case report. J Clin Diagn Res. 2016. 10: PD01-2
8. Gurelik M, Aslan A, Gurelik B, Ozum U, Karadag O, Kars HZ. A safe and effective method for treatment of chronic subdural haematoma. Can J Neurol Sci. 2007. 34: 84-7
9. Han MH. Predictive factors for recurrence and clinical outcomes in patients with chronic subdural hematoma. J Neurosurg. 2017. 127: 1117-25
10. Hasegawa S, Matsumoto J, Ohta K, Fujimoto K, Imaoka Y, Miura M. The beneficial effect of saireito for chronic subdural hematoma. J Neurosurg Kampo Med. 2015. 1: 7-12
11. Hiroyuki T, Keita K, Satoshi H, Hiroki T, Keijiro H, Nobuhisa M. Present epidemiology of chronic subdural hematoma in Japan: Analysis of 63,358 cases recorded in a national administrative database. J Neurosurg. 2008. 128: 222-8
12. Huang YH. Volume of chronic subdural haematoma: Is it one of the radiographic factors related to recurrence?. Injury. 2014. 45: 1327-31
13. Imaizumi T, Horita Y, Honma T, Niwa J. Association between a black band on the inner membrane of a chronic subdural hematoma on T2*-weighted magnetic resonance images and enlargement of the hematoma. J Neurosurg. 2003. 99: 824-30
14. Ishibashi A, Yokokura Y, Adachi H. A comparative study of treatments for chronic subdural hematoma: Burr hole drainage versus burr hole drainage with irrigation. Kurume Med J. 2011. 58: 35-9
15. Ishikawa T, Endo K, Endo Y, Sato N, Ohta M. Neuro-endoscopic surgery for multi-lobular chronic subdural hematoma. No Shinkei Geka. 2017. 45: 667-75
16. Kim CH, Song GS, Kim YH, Kim YS, Sung SK, Son DW. Remote hemorrhage after burr hole drainage of chronic subdural hematoma. Korean J Neurotrauma. 2017. 13: 144-8
17. Kurokawa Y, Ishizaki E, Inaba K. Bilateral chronic subdural hematoma cases showing rapid and progressive aggravation. Surg Neurol. 2005. 64: 444-9
18. Kuroki T, Katsume M, Harada N, Yamazaki T, Aoki K, Takasu N. Strict closed-system drainage for treating chronic subdural haematoma. Acta Neurochir (Wien). 2001. 143: 1041-4
19. Liu W, Bakker NA, Groen RJ. Chronic subdural hematoma: A systematic review and meta-analysis of surgical procedures. J Neurosurg. 2014. 121: 665-73
20. McClelland S, Hall WA. Postoperative central nervous system infection: Incidence and associated factors in 2111 neurosurgical procedures. Clin Infect Dis. 2007. 45: 55-9
21. Miyagami M, Kagawa Y. Effectiveness of Kampo medicine Gorei-San for chronic subdural hematoma. No Shinkei Geka. 2009. 37: 765-70
22. Rohde V, Graf G, Hassler W. Complications of burr-hole craniostomy and closed-system drainage for chronic subdural hematomas: A retrospective analysis of 376 patients. Neurosurg Rev. 2002. 25: 89-94
23. Sahyouni R, Mahboubi H, Tran P, Roufail JS, Chen JW. Membranectomy in chronic subdural hematoma: Meta-analysis. World Neurosurg. 2007. 104: 418-29
24. Suzuki K, Sugita K, Akai T, Takahata T, Sonobe M, Takahashi S. Treatment of chronic subdural hematoma by closed-system drainage without irrigation. Surg Neurol. 1998. 50: 231-24
25. Yadav YR, Parihar V, Namdev H, Bajaj J. Chronic subdural hematoma. Asian J Neurosurg. 2016. 11: 330-42
26. Youden WJ. Index for rating diagnostic tests. Cancer. 1950. 3: 32-5
27. Zakaraia AM, Adnan JS, Haspani MS, Naing NN, Abdullah JM. Outcome of 2 different types of operative techniques practiced for chronic subdural hematoma in Malaysia: An analysis. Surg Neurol. 2008. 69: 608-15