- Department of Neurosurgery, Burnazian FMBC Research Center of FMBA of Russia, Moscow,
- Department of Neurology, Medical Center of Radiology, Krasnodar, Russian Federation.
Correspondence Address:
Levan Teymurazovich Lepsveridze
Department of Neurosurgery, Burnazian FMBC Research Center of FMBA of Russia, Moscow,
DOI:10.25259/SNI_273_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Levan Teymurazovich Lepsveridze1, Maksim Sergeevich Semenov1, Armen Samvelovich Simonyan1, Salome Zurabovna Pirtskhelava2, Georgy Garikovich Stepanyan1, Lado Kobaevich Imerlishvili1. Burr hole microsurgery in treatment of patients with intracranial lesions: Experience of 44 clinical cases. 21-Aug-2020;11:255
How to cite this URL: Levan Teymurazovich Lepsveridze1, Maksim Sergeevich Semenov1, Armen Samvelovich Simonyan1, Salome Zurabovna Pirtskhelava2, Georgy Garikovich Stepanyan1, Lado Kobaevich Imerlishvili1. Burr hole microsurgery in treatment of patients with intracranial lesions: Experience of 44 clinical cases. 21-Aug-2020;11:255. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10220
Abstract
Background: Modern technical capabilities have made minimally invasive surgery increasingly popular. Small incisions can reduce surgical duration and the degree of tissue trauma, which reduces the risk of complications. Burr hole microsurgery is a relatively new minimally invasive technique used in neurosurgery. The objective of this study was to assess the feasibility and outcomes of using burr hole microsurgery for the management of intracranial lesions.
Methods: Forty-four adults were treated with burr hole microsurgery. Patients were divided into groups according to the presence of (1) brain tumors (n = 20); (2) congenital brain cysts (n = 16); (3) cavernous angiomas (n = 3); and (4) neurovascular conflicts of the 5th cranial nerve (n = 5). All surgical interventions were performed using the “MARI” device.
Results: The transcortical approach was used to remove 16 brain tumors, and 2 brain tumors were biopsied. In the two tumor biopsy cases, the parasagittal interhemispheric route was used. Gross total resection was achieved in 10 cases (62.5%) when tumor size reached up to 4 cm, subtotal resection was achieved in four cases (25%) in large tumors, and partial resection in two cases (12.5%). In patients with congenital cysts, cavernous angiomas, trigeminal neuralgia, and symptomatic regression were noted the postoperative period. The surgical duration was 30–180 min (median, 75 min). A hemorrhagic complication was observed in one case. Significant postoperative complications and mortality were not observed.
Conclusion: Burr hole microsurgery can treat different intracranial lesions effectively. Despite a smaller craniotomy diameter of 11–14 mm compared with keyhole approaches, surgery was successful.
Keywords: Brain tumor, Burr hole, MARI device, Microvascular decompression, Minimally invasive surgery, Third ventriculostomy
INTRODUCTION
Minimally invasive brain surgery is possible throughout advances in modern neurosurgery. The past century was marked by the emergence of innovative solutions, such as an operating microscope and updated endoscopic systems, which predetermined the rapid development of small incisions and microsurgery in general. The progressive introduction of “keyhole” incisions in clinical practice was due to Perneczky, a pioneer in endoscopic and minimally invasive microsurgery.[
The MARI device, which was introduced into practical neurosurgery by Pitskhelauri, has made it possible to define new approaches to microsurgery. The MARI device provides the same control capabilities as an operating microscope, where the surgeon’s hands are free from adjusting the microscope and the optical field settings during manipulation.[
In this paper, we present our own experience with burr hole surgery in treating patients with a heterogeneous group of pathologies: intracranial tumors, brain cavernomas, congenital intracranial cysts, and neurovascular conflicts in cranial nerves.
MATERIALS AND METHODS
From January 2019 to November 2019 in our medical center, 44 surgical interventions were performed using the burr hole technique. The age of patients ranged from 25 to 68 years (median, 46 ± 1 year). Patients were divided into the following surgical groups: (1) brain tumors (n = 20, 45%); (2) congenital brain cysts (n = 16, 37%); (3) cavernous angiomas (n = 3, 7%); and (4) neurovascular conflicts of the 5th cranial nerve (n = 5, 11%) [
The inclusion criteria were as follows: (1) intracranial tumors (including metastatic lesions), except for convex and subcortically localized tumors of >2 cm, skull base tumors, and and insular and brainstem lesions; (2) symptomatic hemispheric cavernous angiomas, except for convex cavernomas; (3) symptomatic congenital cysts in different brain regions; and (4) trigeminal neuralgia and magnetic resonance positive forms of neurovascular conflict.
Operating room equipment and instrumentation
Surgery was performed using an OPMI® Pentero® 800 microscope (Zeiss, Germany). In the entire series, surgery was performed using the MARI device. In some cases (9%), a neuronavigation system (BrainLab, Germany) was used if it was necessary to perform open biopsy on various parts of the tumor. Microsurgical sets were presented by standard bayonet instrumentation (i.e., bipolar cautery, tumor grasping forceps, microdissectors, microscissors, and suction tubes with adjustable aspiration intensities and diameters of 2.5, 3.5, and 4.5 mm [Aesculap, Germany]).
Burr hole technique
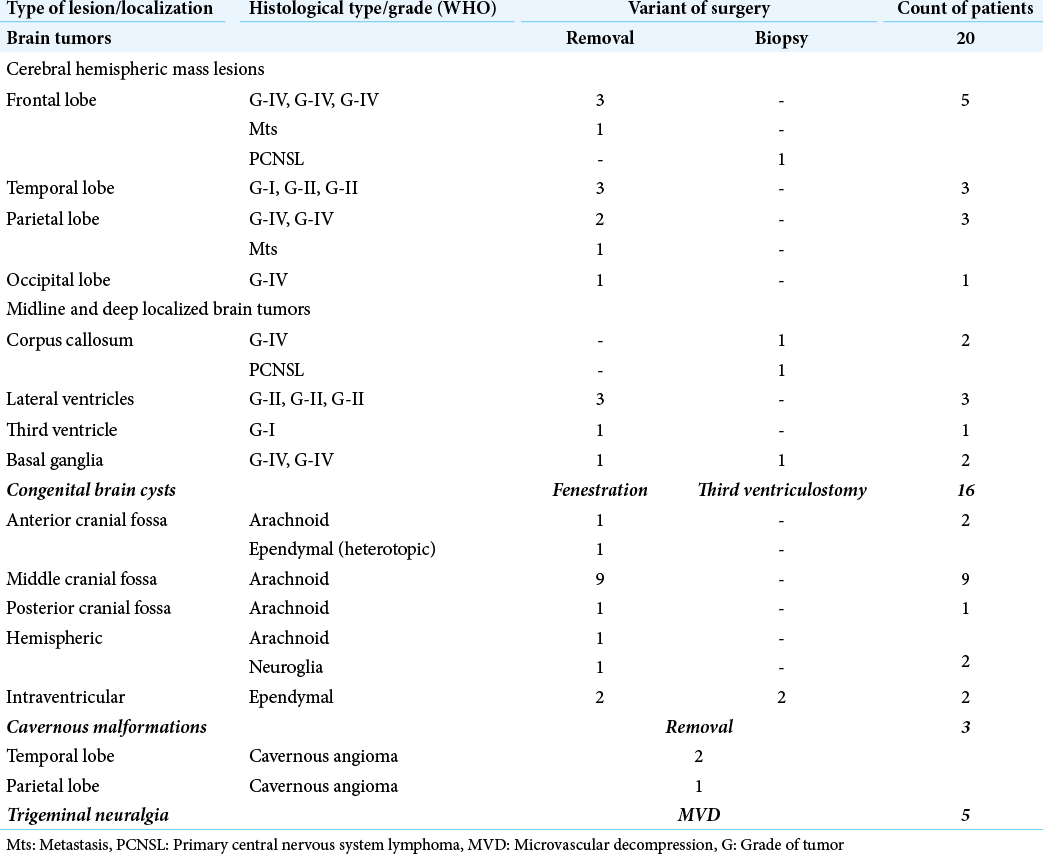
The patient was positioned on the operating table, in accordance with common neurosurgical standards, which were determined by localization of the pathological process. In this series, the sitting position was not used. Skin incisions were linear or C shaped with a 3–4 cm diameter. The “bone stage” was performed using a craniotome (Aesculap, Germany). The skull window diameter varied from 11 to 14 mm depending on the specific craniotome nozzle used [
After craniotomy, a cone-shaped extension of the internal cortical bone around the trephination window was performed using Kerrison-type bone nippers so that the bone window’s internal diameter increased to 15–18 mm depending on the initial trephination window size (11 or 14 mm). After opening with a pointed mini scalpel, the dura mater was sutured along the edge of the trephination with 5/0 threads. In the bulk of cases, an X-shaped dural opening was performed, except for parasagittal and retrosigmoid approaches, where a C-shaped incision was used.
At the end of the main stage of surgery, the dura mater was sutured at its central department with one node using the aforementioned suture material. The dura mater was sealed by applying the TachoComb® (Takeda Austria GmbH) surgical sponge or the hemostatic sponge with fibrin glue. The trephination window was closed using a 20 mm titanium CranioFix® clamp (Aesculap, Germany). The skin incision was sutured with 3/0 thread and an atraumatic needle.
Surgical treatment
Forty-four surgical interventions were performed using the burr hole technique. To remove intracerebral tumors [
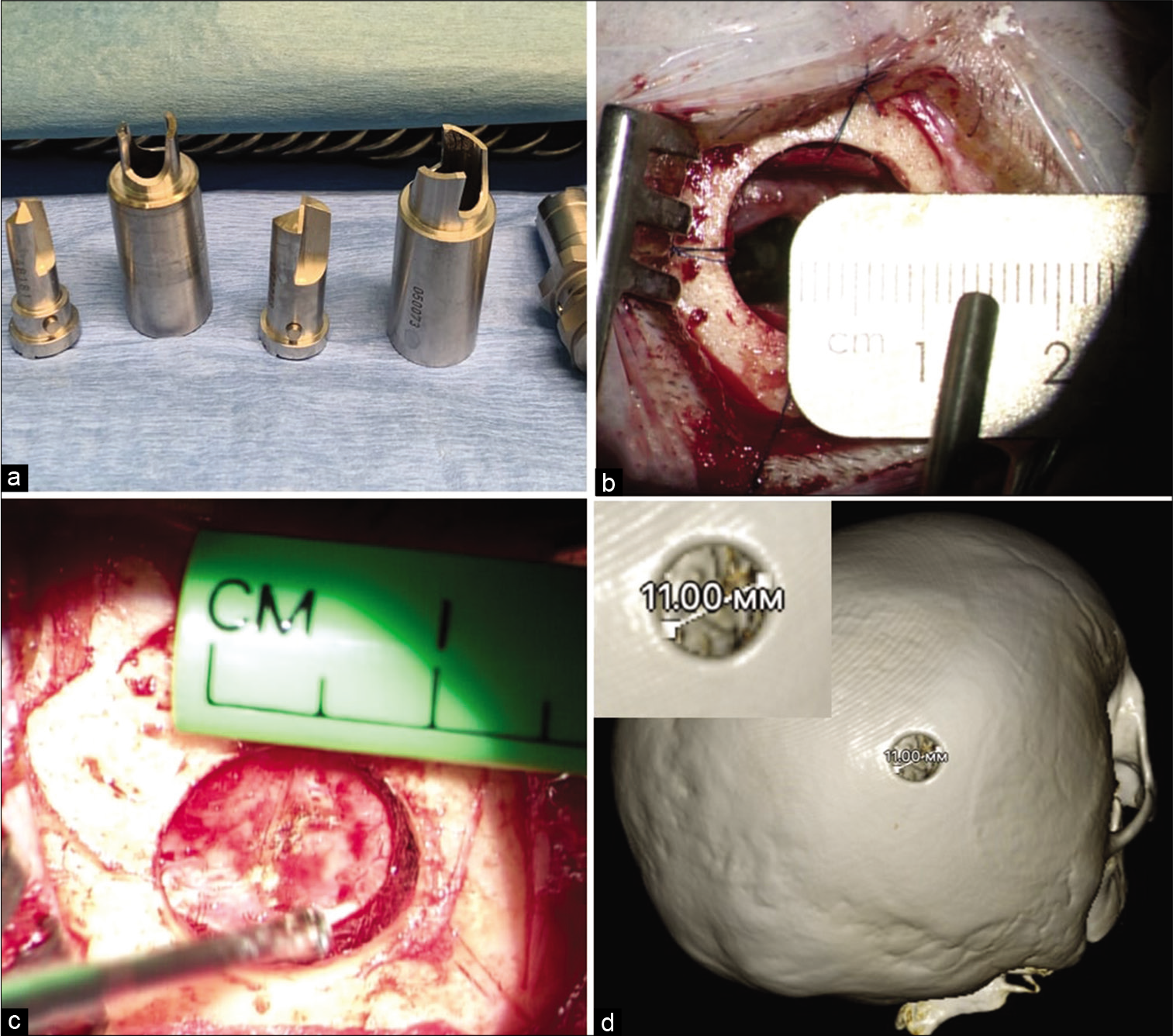
Figure 2:
Removal of glioblastoma of the right hemisphere with spreading into the corpus callosum, through the transcortical approach. (a) The type of planned skin incision. (b and c) MRI before surgery. (d) 3D CT reconstruction of the skull after surgery. (e and f) MRI control on the 1st day after surgery.
Aspiration tubes with diameters of 3.5–4.5 mm allow visualization of the surgical field in different modes and removal of low-density portions of tumors. Hemostasis in the bed of removed tumors was performed using surgical gauze. In rare cases, after removal of hypervascularized tumors and tumors with a swallowed parenchyma with severe venous hypertension, when standard parenchymal hemostasis was difficult, composite fibrin–gelatin complexes were used.
All patients with intracranial congenital cysts underwent surgery for severe cephalalgic syndrome (an average of 7 points on the VAS). Patients participated in >1.5 years of follow-up during the preoperative period, during which they consulted with a neurologist and were examined by an optometrist and a neuropsychiatrist. The surgical group consisted exclusively of patients with symptomatic congenital cysts who noted no positive effects from conservative therapy. The presence of a mass effect and impaired cerebrospinal fluid dynamics, according to fast imaging employing steady- state acquisition (FIESTA) magnetic resonance imaging (MRI) (i.e., phase-contrast MRI with pulse synchronization), was noted in all 16 surgical cases. In these cases, surgery was performed within a diameter of 11–12 mm using small milling cutters.
Depending on the cysts’ topographical and anatomical characteristics, and the involvement of spaces significant for CSF flow (e.g., skull base cisterns and ventricular system of the brain), the following types of fenestration were performed: (1) cystocisternostomy (n = 12) for cysts involving the skull base, when it was possible to conduct a wide fenestration with drainage of the cyst into a large cistern; (2) cystoventriculostomy (n = 2) in cases of hemispheric cysts, when it was necessary to drain the cyst into the ventricular system; (3) ventriculo- cysto-cisternostomy (n = 2), where cyst fenestration was performed after ventricular access with simultaneous microsurgical third ventriculostomy.
Cyst fenestration was performed using standard microsurgical instruments, mainly with an acute approach. During surgery on brain cysts, in conditions of small access, suction tubes with a small diameter (~2.5 mm) were used, since adequate manipulation of two instruments simultaneously was difficult. In two cases of intraventricular lesions with occlusive hydrocephalus, microsurgical third ventriculostomies were performed. In all arachnoid cyst cases, there was high cerebrospinal fluid pressure after the cystic membrane was opened. Intraoperative biopsies and histological examinations were performed in 100% of patients. The biopsy material was a shell of the arachnoid cyst or a portion of the cystic tissue in cases of intracerebral cysts. After fenestration, closure of the dura mater and bone window was carried out using a similar approach to that used in brain tumor surgery.
Burr hole surgery was carried out in patients with cavernous angiomas in the temporal lobe and neurovascular conflict in the roots of the 5th cranial nerve [
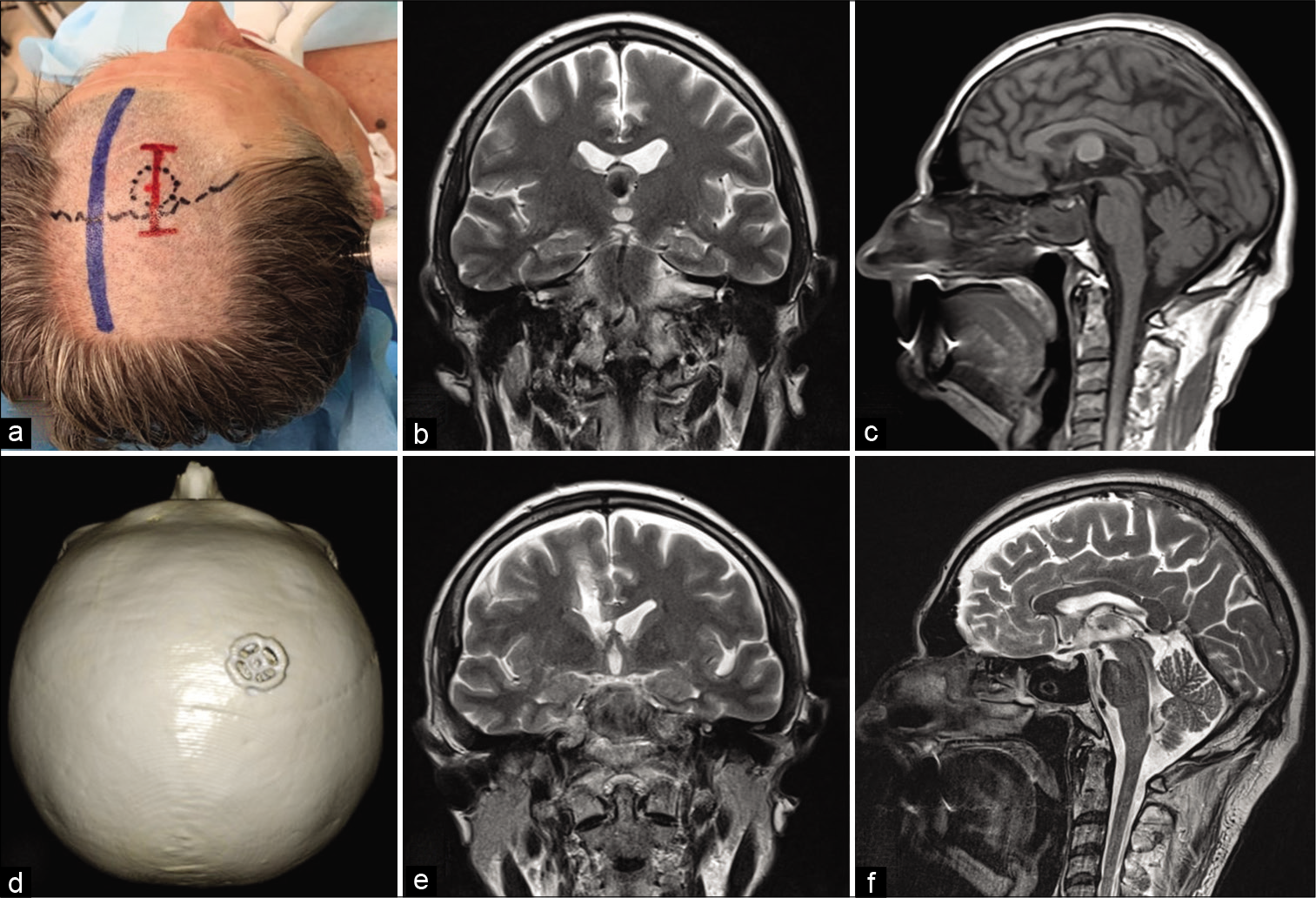
Figure 4:
Microvascular decompression of the left trigeminal nerve. (a) The patient is positioned in a park bench. (b) The short line of skin incision for left-sided retrosigmoid burr hole approach. (c) Left 5th nerve after decompression (compression of 5th nerve by the anterior inferior cerebellar artery). (d) CT image after mini burr hole craniotomy. (e) View of Titanium CranioFix® after burr hole closure. (f) 3D CT reconstruction of the skull after surgery.
RESULTS
Forty-four patients underwent burr hole microsurgery. Twenty patients with intracranial mass lesions underwent surgery. A transhemispheric transcortical approach was performed to remove 16 tumors (80%), while tumor biopsies were performed in 2 patients (10%). A parasagittal interhemispheric tumor biopsy was performed on 2 patients (10%) with primary central nervous system lymphoma and malignant glioma of the corpus callosum.
In tumor removal cases, a high radicality and total removal were observed in 62.5% of patients where the tumor size reached up to 4 cm. Subtotal tumor removal was achieved in 25% of patients, mainly with large tumors, while partial tumor removal was achieved in 12.5% of patients. The postoperative hospital stay was 5–9 days. Difficulty in achieving hemostasis due to narrow access was not noted in our study.
In all patients with congenital cysts, cephalalgic syndrome regression was noted (in 75% of patients with 0 points on the VAS and in 25% of patients with 2–3 points on the VAS). In patients with cavernous angiomas, complete regression of paroxysmal symptoms occurred. Repeated seizures in the postoperative period were not observed in patients with prophylactic antiepileptic drug use. The follow-up period in this group of patients was 4–6 months.
Patients with trigeminal neuralgia noted symptomatic regression without functional impairment in sensitivity. The surgical duration, from the beginning of the skin incision to suturing closure, was within 30–180 min (median, 75 min). The shortest surgical duration was observed in patients with arachnoid cysts in the brain. The longest surgical duration was observed in patients with intracerebral tumors of >4 cm. Postoperative cerebrospinal fluid leakage was not observed. Postoperative mortality was also not noted.
Complications
Hemorrhagic complications were observed in one patient with hydrocephalus due to occlusion in the oral part of the cerebral aqueduct. After microsurgical third ventriculostomy, an ipsilateral subdural hematoma was noted in postoperative images. In this patient, during dynamic observation, the hematoma regressed, and repeated intervention was not required. Postoperative cerebrospinal fluid leakage was not observed. No fatalities were reported.
DISCUSSION
Minimally invasive microsurgery is a progressive solution for treating patients with various intracranial pathologies. Minimally invasive methods in neurosurgical practice underwent positive changes with the advent of modern endoscopic devices and high-resolution microscopes.[
cerebral structures, such as the third ventricle, pineal region, insular lobe, and brainstem, can now be safely subjected to surgery.[
Burr hole microsurgery is a recent trend in minimally invasive intracranial surgery.[
In all patients with intracranial tumors, the resection radicality remains high, which provides a positive view of this technique. The most commonly used transcortical approach is associated with minor trauma to associative and commissural fibers close to functionally significant areas of the brain.[
Full fenestration of cysts was performed through 11 mm access. No cases required conversion to a standard craniotomy or endoscopic assistance. Microsurgical third ventriculostomy makes it possible to solve liquorodynamic problems associated with intraventricular invasion. Regarding to neurovascular conflicts and cavernomas, the technique allows the possibility of small access on vascular formations with different complexities of arachnoid dissection in the projection of arteries and veins.
At this stage, due to the short observation period, the small sample size of 44 patients, and the heterogeneity of lesions examined, we did not conduct a comparative analysis with standard microsurgery.
As experience is gained, we hope to conduct a deeper analysis of the method and present it in future publications.
CONCLUSION
The minimally invasive burr hole approach can provide high-quality surgery to treat various types of intracranial lesion. Despite the small craniotomy diameter (11–14 mm) compared with keyhole approaches, surgery was successful.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Badie B, Brooks N, Souweidane MM. Endoscopic and minimally invasive microsurgical approaches for treating brain tumor patients. J Neurooncol. 2004. 69: 209-19
2. Choque-Velasquez J, Resendiz-Nieves J, Jahromi BR, Colasanti R, Baluszek S, Muhammad S. Midline and paramedian supracerebellar infratentorial approach to the pineal region: A comparative clinical study in 112 patients. World Neurosurg. 2020. 137: e194-207
3. Cikla U, Swanson KI, Tumturk A, Keser N, Uluc K, Cohen-Gadol A. Microsurgical resection of tumors of the lateral and third ventricles: Operative corridors for difficult-to-reach lesions. J Neurooncol. 2016. 130: 331-40
4. Cohen AR. Endoscopic ventricular surgery. Pediatr Neurosurg. 1993. 19: 127-34
5. Decq P, Le Guerinel C, Brugières P, Djindjian M, Silva D, Kéravel Y. Endoscopic management of colloid cysts. Neurosurgery. 1998. 42: 1288-94
6. Dye JA, Dusick JR, Lee DJ, Gonzalez NR, Martin NA. Frontal bur hole through an eyebrow incision for image-guided endoscopic evacuation of spontaneous intracerebral hemorrhage. J Neurosurg. 2012. 117: 767-73
7. Gazzeri R, Nalavenkata S, Teo C. Minimally invasive key-hole approach for the surgical treatment of single and multiple brain metastases. Clin Neurol Neurosurg. 2014. 123: 117-26
8. Iacoangeli M, Nocchi N, Nasi D, Di Rienzo A, Dobran M, Gladi M. Minimally invasive supraorbital key-hole approach for the treatment of anterior cranial fossa meningiomas. Neurol Med Chir (Tokyo). 2016. 56: 180-5
9. Kassam AB, Engh JA, Mintz AH, Prevedello DM. Completely endoscopic resection of intraparenchymal brain tumors. J Neurosurg. 2009. 110: 116-23
10. Macgregor BJ, Gawler PJ, South JR. Intracranial epithelial cysts. Report of two ceases. J Neurosurg. 1979. 44: 195-8
11. Perneczky A, Fries G. Endoscope-assisted brain surgery: Part 1--evolution, basic concept, and current technique. Neurosurgery. 1998. 42: 219-24
12. Pitskhelauri D, Konovalov A, Kudieva E, Bykanov A, Pronin I, Eliseeva N. Burr hole microsurgery for intracranial tumors and mesial temporal lobe epilepsy: Results of 200 consecutive operations. World Neurosurg. 2019. 126: e1257-67
13. Pitskhelauri D, Konovalov A, Shekutev G, Rojnin N, Kachkov I, Samborskiy D. A novel device for hands-free positioning and adjustment of the surgical microscope. J Neurosurg. 2014. 121: 161-4
14. Ruge JR, Johnson RF, Bauer J. Burr hole neuroendoscopic fenestration of quadrigeminal cistern arachnoid cyst: Technical case report. Neurosurgery. 1996. 38: 830-7
15. Schroeder HW, Gaab MR, Niendorf WR. Neuroendoscopic approach to arachnoid cysts. J Neurosurg. 1996. 85: 293-8
16. Souweidane MM. Endoscopic management of pediatric brain tumors. Neurosurg Focus. 2005. 18: E1
17. Tamburrini G, Dal Fabbro M, Di Rocco C. Sylvian fissure arachnoid cysts: A survey on their diagnostic workout and practical management. Childs Nerv Syst. 2008. 24: 593-604