- Department of Surgical Neuro-Oncology, National Institute of Neurology and Neurosurgery, Mexico City, Mexico.
- Gamma Knife Unit, Medica Sur Foundation, Mexico City, Mexico.
DOI:10.25259/SNI_706_2020
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Javier A. Jacobo1, Masao Buentello2, Ramiro Del Valle2. C-methionine-PET-guided Gamma Knife radiosurgery boost as adjuvant treatment for newly diagnosed glioblastomas. 31-May-2021;12:247
How to cite this URL: Javier A. Jacobo1, Masao Buentello2, Ramiro Del Valle2. C-methionine-PET-guided Gamma Knife radiosurgery boost as adjuvant treatment for newly diagnosed glioblastomas. 31-May-2021;12:247. Available from: https://surgicalneurologyint.com/surgicalint-articles/10836/
Abstract
Background: The most common glial tumor is the glioblastoma, and the prognosis remains dismal despite a multimodal therapeutic approach. The role of radiosurgery for the treatment of glioblastomas has been evaluated in several studies with some benefit at the recurrent stage. We evaluate the results of the protocol administered at the Gamma Knife unit administering radiosurgery as a boost to metabolic active parts of the tumor after the patient had completed traditional external beam radiotherapy (XBRT) as part of the Stupp protocol for high-grade gliomas.
Methods: This is a retrospective analysis of seven patients with newly diagnosed glioblastomas who were treated with Gamma Knife radiosurgery as a boost after receiving XBRT as part of the Stupp protocol. The target of radiation was determined according to the findings of the C-methionine PET scan in relation to magnetic resonance images. The primary end point of this study was to determine the progression-free survival (PFS) from the time of diagnosis.
Results: The median age of patients was 48.8 years and the mean Karnofsky performance score was 92.8%. The median PFS was 12.4 months. No radiation adverse effects were documented.
Conclusion: Stereotactic radiosurgery is safe to use in the upfront treatment for these patients and appears to have a beneficial role in improving the PFS. This beneficial role seems to be conditioned not only by the time the treatment is administered but also where the radiation dose is targeted to.
Keywords: Boost, Glioblastoma, Radiosurgery, Upfront
INTRODUCTION
Gliomas are the most common primary central nervous system tumors, with an estimated annual incidence of 6.6/100,000 individuals in the United States.[
Unfortunately, the prognosis in patients with GBM remains dismal despite a multimodal therapeutic approach utilizing maximal safe resection and adjuvant chemo- and radiotherapy.[
The role of stereotactic radiosurgery (SRS) has been evaluated in several studies with poor results at the upfront stage, although some benefit has been found when administered at the recurrent stage.
We evaluate the results of the protocol administered at the Gamma Knife unit administering SRS as a boost to metabolic active parts of the tumor after the patient had completed traditional external beam radiotherapy (XBRT) as part of the Stupp protocol for high-grade gliomas.
MATERIALS AND METHODS
This is a retrospective analysis of seven patients with newly diagnosed GBM who were treated with Gamma Knife SRS as a boost after receiving XBRT as part of the Stupp protocol.
Patients were identified through the record logs of the Hoag Gamma Knife program. Only patients with a histological diagnosis of GBM at original diagnosis were included in the study. All patients underwent craniotomy or stereotactic biopsy for tumor debulking/diagnosis before the radiation treatment. All patients underwent SRS after standard XBRT and temozolomide (TMZ) chemotherapy.
Patients with recurrent GBM, brainstem tumors, multifocal GBM, or gliomatosis cerebri were excluded from the study.
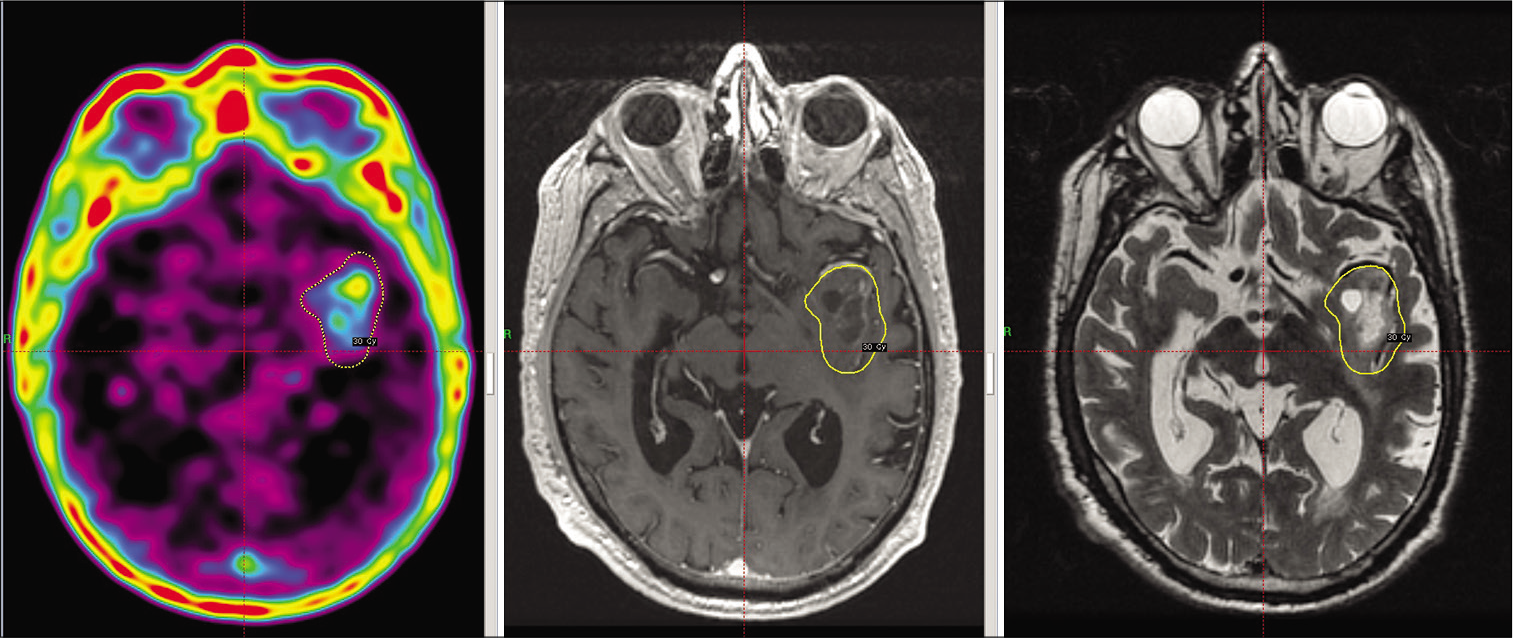
The coregistration of MRI sequences (T1-gadolinuim, T2 and T2 FLAIR) and C-methionine (C-MET)-PET images using the standard chemical shift multivoxel software supplied by the vendor was used to design treatment plans that targeted the area with abnormal metabolic enhancement according to the PET in relation to the anatomical landmarks seen in the MRI [
The dose was prescribed to the 50% isodose line in all cases, using multiple isocenters to encompass the margin of the target.
It was possible to determine the molecular diagnosis of the tumor in four of the patients, this included in some cases IDH1, p53, or EGFR status.
The extent of resection (EOR) of the tumor was measured according to the preoperative MRI in the T1-gadolimium sequences.
It was considered a biopsy if the resection was under 70%, subtotal resection (STR) if the resection was over 70% but under 90%, near-total resection over 90%, and gross total resection (GTR) if the entire enhanced region of the tumor was resected.
The primary end point of this study was progression-free survival (PFS) from the time of diagnosis, given that at the time of progression, treatment modalities for GBM may vary significantly and alter the final analysis of the overall survival (OS).
Tumor recurrence was documented according to the RANO criteria at follow-up MRI that was performed every 3 months after the radiosurgical procedure.
RESULTS
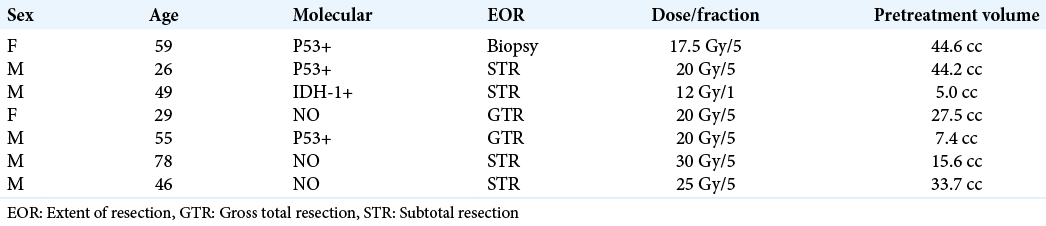
Seven patients met the inclusion criteria for the study. The median age of patients was 48.8 years (range 26–78) and the mean Karnofsky performance score was 92.8 (range 80–100).
One patient had an IDH-1 mutant GBM, three patients had GBMs positive for p53 mutation. The other three patients had no information regarding the molecular diagnostic status.
According to the established EOR parameters, one patient received a biopsy only, four patients received a STR of the tumor, and a near-total resection was performed in two patients.
All patients received standard XBRT of 60 Gy in 30 sessions and concomitant TMZ 1–4 months before the boost treatment with Gamma Knife SRS.
The median target volume was 25.4 cm3 (range 5–44.6 cm3). The radiation doses varied according to the volume; one patient received a single dose of 12 Gy, four patients received 20 Gy in 5 fractions, one patient received 25 Gy in 5 fractions, and the last patient received 30 Gy in 5 fractions.
[
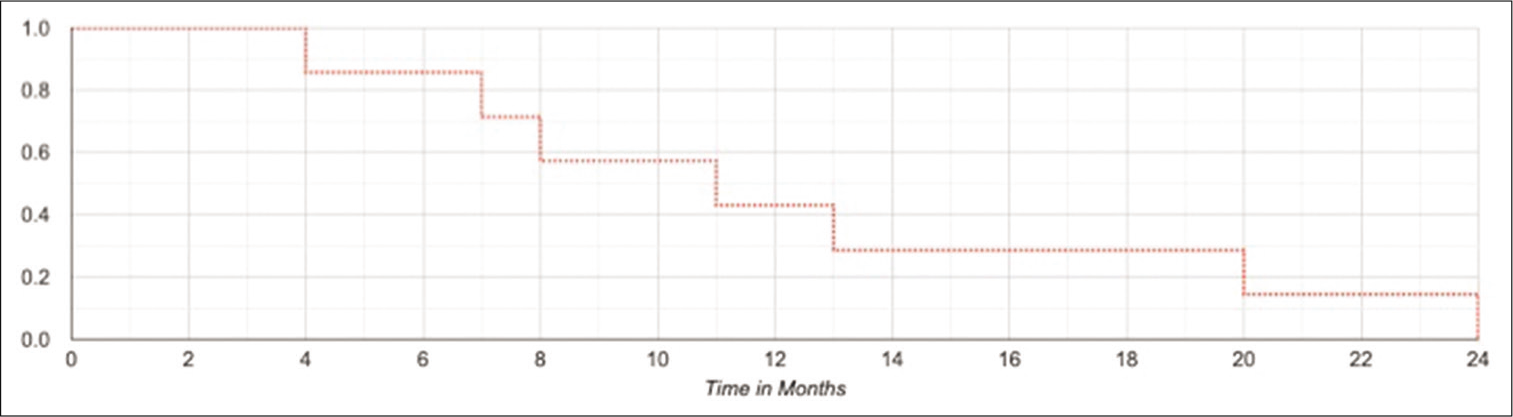
The median PFS was 12.4 months (range 4–24). The 6, 12, 18, and 24 months PFS were 85%, 42%, 28%, and 14%, respectively [
[
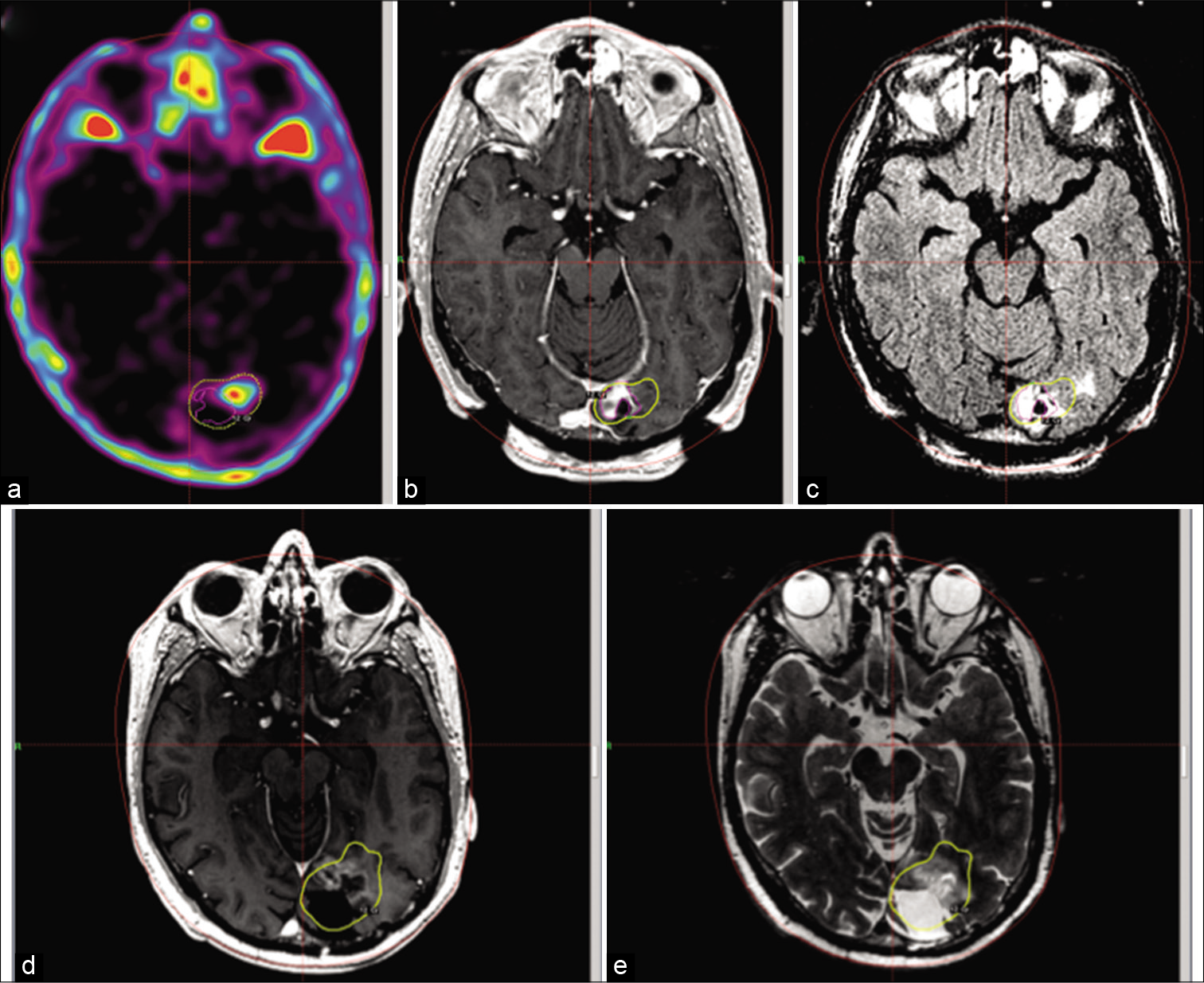
Figure 3:
C-methionine-PET (a), T1-gadolinium (b), and T2 FLAIR (c) images at the time of the stereotactic radiosurgery treatment. The treatment margin is extended laterally from where contrast enhancement is seen, as guided by the hypermetabolic region in the PET. Follow-up images (d and e) at 1 year, no abnormal imaging is seen outside the margin dose.
No adverse effects attributed to the radiation treatment were documented in any of the patients.
DISCUSSION
The current standard treatment for GBM includes maximal safe resection followed by XBRT and chemotherapy with TMZ.[
In 2016, the WHO established a new classification for glial tumors that aside from the histological characteristics, included molecular genetics as a form to better understand their behavior.[
We mention that the PFS for these patients reaches up to 24 months, but it is important to state that most of the patients, due to limitations in our current health system, do not have a complete molecular diagnosis. The information that we do have shows that one patient was positive for IDH-1 mutation and other three patients were positive for p53 mutation. A positive relation between IDH-1 mutation and p53 mutation has been found in the past studies.[
The appropriate timing of SRS in the treatment of GBM is also an issue that has been investigated in several studies. These studies have suggested that Gamma Knife SRS is more appropriate as a treatment option for recurrent tumors than as an upfront radiosurgical boost.[
Functional nuclear imaging may be used to investigate metabolism-related changes for oncological response assessment in gliomas.[
Contrary to 18F-FDG, C-MET has a great sensitivity and specificity to identify malignant lesions from benign ones, and additionally, its binding highly correlates with the proliferation index.[
In 2016, a series of recommendations for the clinical use of PET imaging in gliomas was published, given birth to the PET-RANO guidelines.[
Of particular interest, the implication of C-MET PET for the identification of tumor extent for resection planning or radiotherapy planning was mentioned.
This affinity that C-MET presents for GBM makes it a great tool to guide the radiosurgical procedure, and we believe serves as a better target than to disperse the radiation dose on a greater volume or to focus the dose in parts of the tumor where it would not be useful.
At the moment, the accepted initial treatment for patients with newly diagnosed GBM includes maximal safe resection followed by XBRT and chemotherapy with TMZ.[
At the time of recurrence, patients will require new treatment approaches with new surgeries and different immune and chemoradiation treatments,[
Even though the number of patients in our case series is low, we have showed that the addition of PET-guided SRS after conventional XBRT may have a beneficial impact on the PFS of patients with GBM. Extending the need for further treatments to over a year improves greatly the quality of life and also the performance status for these patients because it delays the neurological deterioration related to tumor progression.
Another thing to keep in mind is that adding SRS to the initial treatment does not interfere with the possible treatments at the time of progression, this includes a second session of SRS in the future, which have also shown to improve the OS for patients with recurrent GBM.[
At the time of last follow-up visit, we have not documented radiation-induced adverse effects, including radionecrosis. In the literature, the incidence for radionecrosis after SRS for newly diagnosed GBM varies from 0% to 38%, and greater series with a greater margin dose report the highest numbers.[
This study has limitations; the retrospective nature of the study, the low number of patients that we were able to enroll, and the lack of control group should make the reader cautious about the interpretation of the results.
Nevertheless, we believe that this could open doors for further studies with better methodologies and reopen the door for the inclusion of SRS in the upfront treatment of patients with newly diagnosed GBM.
CONCLUSION
Patients with GBM have a short survival despite advances in the treatment modalities of this disease.
SRS is a feasible and safe technique to use in the upfront treatment for patients with newly diagnosed GBM and we hypothesize that it may have a beneficial role in improving the PFS and also the OS as demonstrated by other studies. This beneficial role seems to be conditioned not only by the time the treatment is administered but also where the radiation dose is targeted to.
Further studies are needed to confirm this hypothesis.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Albert NL, Weller M, Suchorska B, Galldiks N, Soffietti R, Kim MM. Response assessment in neuro-oncology working group and European association for neuro-oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 2016. 18: 1199-208
2. Birner P, Toumangelova-Uzeir K, Natchev S, Guentchev M. Expression of mutated isocitrate dehydrogenase-1 in gliomas is associated with p53 and EGFR expression. Folia Neuropathol. 2011. 49: 88-93
3. Botros D, Dux H, Price C, Khalafallah AM, Mukherjee D. Assessing the efficacy of repeat resections in recurrent glioblastoma: A systematic review. Neurosurg Rev. 2020. 2020: 01331-1
4. Chen C, Damek D, Gaspar LE, Waziri A, Lillehei K, Kleinschmidt-DeMasters BK, Robischon M. Phase I trial of hypofractionated intensitymodulated radiotherapy with temozolomide chemotherapy for patients with newly diagnosed glioblastoma multiforme. Int J Rad Oncol Biol Phys. 2011. 81: 1066-74
5. Chung JK, Kim YK, Kim SK, Lee YJ, Paek S, Yeo JS. Usefulness of 11C-methionine PET in the evaluation of brain lesions that are hypo-or isometabolic on 18F-FDG PET. Eur J Nucl Med Mol Imaging. 2002. 29: 176-82
6. Crocetti E, Trama A, Stiller C, Caldarella A, Soffietti R, Jaal J. Epidemiology of glial and non-glial brain tumours in Europe. Eur J Cancer. 2012. 48: 1532-42
7. Duma CM, Kim BS, Chen PV, Plunkett ME, Mackintosh R, Mathews MS, Casserly RM. Upfront boost Gamma Knife “leading-edge” radiosurgery to FLAIR MRI-defined tumor migration pathways in 174 patients with glioblastoma multiforme: A 15-year assessment of a novel therapy. J Neurosurg. 2016. 125: 40-9
8. Faustino AC, Viani GA, Hamamura AC. Patterns of recurrence and outcomes of glioblastoma multiforme treated with chemoradiation and adjuvant temozolomide. Clinics (Sao Paulo). 2020. 75: e1553
9. Fiveash JB, Spencer SA. Role of radiation therapy and radiosurgery in glioblastoma multiforme. Cancer J. 2003. 9: 222-9
10. Gigineishvili D, Shengelia N, Shalashvili G, Rohrmann S, Tsiskaridze A, Shakarishvili R. Primary brain tumour epidemiology in Georgia: First-year results of a population-based study. J Neurooncol. 2013. 112: 241-6
11. Gilbert MR, Wang M, Aldape KD, Stupp R, Hegi ME, Jaeckle A. Dose-dense temozolomide for newly diagnosed glioblastoma: A randomized phase III clinical trial. J Clin Oncol. 2013. 31: 4085-91
12. Hsieh PC, Chandler JP, Bhangoo S, Panagiotopoulos K, Kalapurakal JA, Marymont MH. Adjuvant gamma knife stereotactic radiosurgery at the time of tumor progression potentially improves survival for patients with glioblastoma multiforme. Neurosurgery. 2005. 57: 684-92
13. Latifyan S, de Micheli R, Hottinger AF. Physical approaches to treat glioblastoma. Curr Opin Oncol. 2020. 32: 640-9
14. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK.editors. WHO Classification of Tumours of the Central Nervous System. Lyon, France: International Agency for Research on Cancer; 2016. p. 10-122
15. Moreau A, Febvey O, Mognetti T, Frappaz D, Kryza D. Contribution of different positron emission tomography tracers in glioma management: Focus on glioblastoma. Front Oncol. 2019. 9: 1134
16. Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C. CBTRUS statistical report: primary brain and central nervous system tumours diagnosed in the United States in 2008-2012. Neuro Oncol. 2015. 17: iv1-62
17. Shah JL, Li G, Shaffer JL, Azoulay MI, Gibbs IC, Nagpal S. Stereotactic radiosurgery and hypofractionated radiotherapy for glioblastoma. Neurosurgery. 2018. 82: 24-34
18. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005. 352: 987-96
19. Verger A, Langen KJ, De Vleeschouwer S.editors. PET imaging in glioblastoma: Use in clinical practice. Glioblastoma. Brisbane, AU: Codon Publications; 2017. 9:
20. Vordermark D, Kölbl O. Lack of survival benefit after stereotactic radiosurgery boost for glioblastoma multiforme: Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: Report of radiation therapy oncology group 93-05 protocol: In regard to Souhami et al. (Int J Radiat Oncol Biol Phys 2004;60:853-860). Int J Radiat Oncol Biol Phys. 2005. 62: 296-7