- Department of Orthopaedics, University of Rochester, Rochester, New York, United States.
Correspondence Address:
Addisu Mesfin, Department of Orthopaedics, University of Rochester, Rochester, New York, United States.
DOI:10.25259/SNI_684_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mark Ehioghae, Mark C. Lawlor, Addisu Mesfin. Calcium pyrophosphate dihydrate of the ligamentum flavum in the cervical spine – A review of the literature. 14-Oct-2022;13:470
How to cite this URL: Mark Ehioghae, Mark C. Lawlor, Addisu Mesfin. Calcium pyrophosphate dihydrate of the ligamentum flavum in the cervical spine – A review of the literature. 14-Oct-2022;13:470. Available from: https://surgicalneurologyint.com/surgicalint-articles/calcium-pyrophosphate-dihydrate-of-the-ligamentum-flavum-in-the-cervical-spine-a-review-of-the-literature/
Abstract
Background: Calcium pyrophosphate dihydrate (CPPD) deposition, also known as pseudogout, in the cervical ligamentum flavum (CLF), is a rare disease which can cause spinal cord signaling changes leading to rapid deterioration in function. The natural history of cervical myelopathy as a result of CPPD deposition within the CLF is not well understood. Our objective is to describe the presentation, imaging findings, and treatment options of CPPD deposition or pseudogout of the cervical spine.
Methods: Using PubMed, we analyzed studies published from 1978 to 2022. Key words used were “pseudogout,” “CPPD deposit disease,” “cervical yellow ligament,” “CLF,” and “cervical spine.” We excluded “crowned dense syndrome” and “ossification of ligament flavum.” Using a department database, we queried for patients treated for CPPD of the cervical spine.
Results: Twenty clinical studies on CPPD of the cervical spine with 69 patients aged between 15 and 92 years (mean = 72) were identified. Neck pain and numbness of the hands were the most common symptoms. Diabetes mellitus and hypertension were the most common comorbidities. Males and females were affected at equal rates. C4-C5 and C5-C6 were the most affected segments. Earlier surgical treatment produced better outcomes. A laminectomy and fusion or laminoplasty were the most common procedures performed with most patients experiencing some return of neurologic function.
Conclusion: Although rare, CPPD deposit disease in the CLF should be readily considered as a differential diagnosis due to the continuously aging population. CPPD’s progressively worsening nature makes an early diagnosis and treatment important in improving the patient’s overall quality of life.
Keywords: Calcium pyrophosphate dihydrate, Cervical fusion, Cervical laminectomy, Cervical spine, Cervical stenosis, Laminoplasty, Ligamentum flavum, Myelopathy
INTRODUCTION
Calcium pyrophosphate dihydrate (CPPD) deposit disease, also known as pseudogout and formerly known as articular chondrocalcinosis, was first defined by Zitnan and Sit’aj in 1958.[
The natural history of cervical myelopathy as a result of CPPD deposition within the CLF is not well understood.[
SYNOPSIS OF INCLUDED STUDIES
A total of 20 clinical studies on CPPD deposit disease in the CLF were identified with 69 patients included for analysis. We analyzed all clinical studies published before 2022. The first report was in 1978 and the most recent in 2021. We identified articles using PubMed with references listed as peer-reviewed publications. Key words used included “pseudogout, “CPPD deposit disease,” “cervical yellow ligament,” “CLF,” and “cervical spine.” We excluded “crowned dense syndrome” and “ossification of ligament flavum.”
CASE DESCRIPTIONS
Using the department’s database, two cases of CPPD deposit disease in the CLF were identified. The clinical and histological evidence is discussed.
Case 1
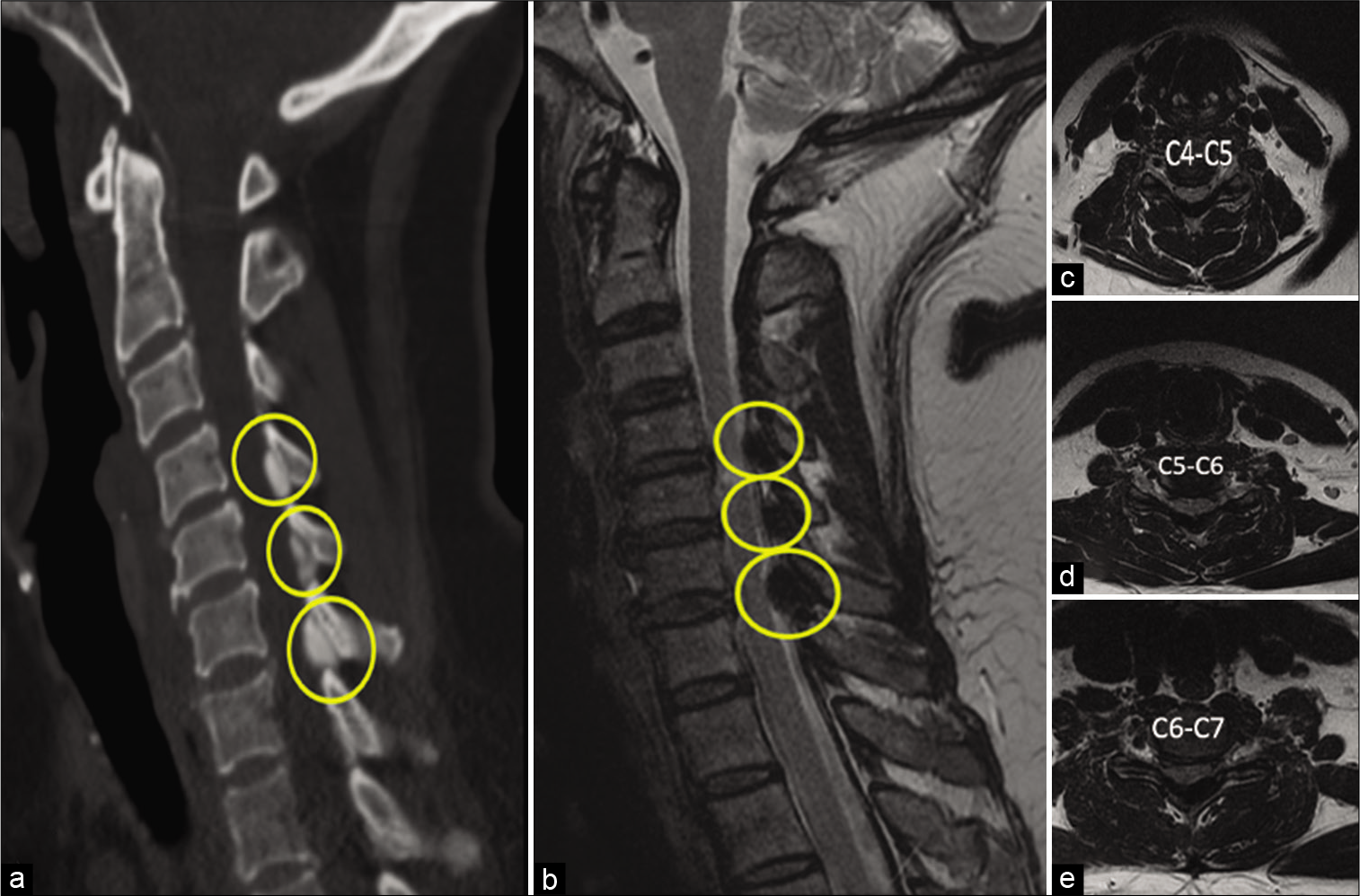
A 60-year-old woman, with cervical myelopathy, presenting with balance and gait abnormalities. Magnetic resonance imaging (MRI) and computed tomography (CT) identified a dorsal lesion compressing the spinal cord emanating from the ligamentum flavum at the C4-C5, C5-C6, and C6-C7 levels [
Figure 1:
Sagittal Computed tomography (a), T2-weighted Magnetic resonance imaging (b), and (c,d,e) Axial T2-weighted images of calcium pyrophosphate dehydrate (CPPD) deposit disease in the cervical ligamentum flavum (CLF) of a 60-year-old female with cervical myelopathy presenting with balance and gait abnormalities. Sagittal yellow images circle (a,b) denotes a CPPD compression lesion in C4-C5, C5-C6, and C6-C7 CLF lamina. Axial images present spinal canal stenosis.
Case 2
A 70-year-old woman, with cervical myelopathy, presenting with balance and gait abnormalities resulting in falls. MRI and CT demonstrated a dorsal based compression of the spinal cord emanating from the ligamentum flavum at the C4-C5 [
Figure 4:
Sagittal computed tomography (CT) (a), T2-weighted magnetic resonance imaging (b), axial CT (c), and T2-weighted (d) images of calcium pyrophosphate dehydrate (CPPD) deposit disease in the cervical ligamentum flavum (CLF) of a 70-year-old female with cervical myelopathy presenting with balance and gait abnormalities experienced falls. The yellow circles (a,b,c,d) denotes a CPPD compression lesion in C4-C5 CLF lamina.
EPIDEMIOLOGY AND RISK FACTORS
Various factors play a role in the development of CPPD in the CLF such as aging, metabolic diseases, mechanical stress, and endocrine imbalance.[
Overall, aging is a major risk factor for CPPD deposit disease in the CLF. The prevalence of CPPD deposit disease increases from 3.7% to 17.5% as age groups increase from 55–59 to 80–84, respectively.[
While CPPD in the CLF has a mean onset age of 72–73 years, on rare occasions, it can be observed in the pediatric population. Morino et al. 2016[
Another risk factor that is commonly reported is gender.[
However, we found similar results as Richette et al. in 2009 with similar prevalence between men and women [
Although the direct cause of pseudogout is still unclear, some reported that metabolic risk factors for CPPD deposit disease in the CLF are hyperthyroidism, hypothyroidism, hyperparathyroidism, osteoarthritis, diabetes mellitus, arthrosclerosis, hypertension and hemochromatosis, Wilson’s disease, hypophosphatasia, hypomagnesemia, and loop diuretics use.[
PATHOLOGY/PATHOPHYSIOLOGY
The cervical spine is an area of high mobility. As such, the ligamentum flavum is highly elastic in this section of the spine leaving it susceptible to microscopic tears.[
The origin of CPPD deposit disease in the CLF is unknown but three factors may influence its deposition: (i) metabolic disorders; (ii) sporadic inheritance; and (iii) familial inheritance.[
Morphologically, the central region of the CLF is the primary area affected as it becomes surrounded by denigrative fibers due to CPPD deposition.[
CPPD is frequently associated with generalized collagen degeneration.[
DIAGNOSIS
The primary methods of diagnosis of CPPD in the CLF are through a combination of symptomatology, MRI, CT, and histopathology.
The symptoms of CPPD deposit disorder are progressive in nature with global worsening and loss of function with time.[
CPPD deposition disease in the CLF is commonly mistaken for ossification of ligamentum flavum (OLF) which is more often centrally identified in the lower thoracic spine and generally localized to a single level.[
CPPD deposition does not extend to the posterior facet joints while OLF always extends to the posterior facet joint. CPPD deposition is not continuous with the lamina and OLF shows continuity with the lamina.[
Although gout and CPPD deposit disease present similarly in their ability to deposit crystals into joints, cartilage, and ligaments causing an inflammatory response,[
IMAGING
Sagittal CT [
Sagittal and axial MRI with T1- and T2-weighted imaging [
CPPD nodules show low signal intensity in T1- and T2-weighted image surrounded by areas of high and medium signal intensity considered an appearance of edematous change. Lu et al. 2021[
MANAGEMENT OF CPPD
As discussed, prognostic factors of CPPD deposition disease in the CLF include age, comorbidities, rate of deterioration, severity and duration of symptoms, and spinal cord signal changes.
Management of CPPD deposition disease in the CLF is dependent on the course of the disease. Although CPPD crystals can cause inflammation, many patients do not present with abnormally high inflammatory markers.[
A laminectomy is a highly effective method of relieving cervical myelopathy caused by CPPD lesion as the symptomatic lesions are typically confined to specific spinal segments.[
Early surgical treatment produces better long-term outcome and return of neurological function.[
CONCLUSION
Although a rare condition, the prevalence of CPPD in the CLF indicates that it should be considered as a differential diagnosis particularly as the age of the population increases. CPPD’s progressively worsening nature makes an early diagnosis and treatment important in improving the patient’s overall quality of life and functional outcome.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Baba H, Maezawa Y, Kawahara N, Tomita K, Furusawa N, Imura S. Calcium crystal deposition in the ligamentum flavum of the cervical spine. Spine (Phila Pa 1976). 1993. 18: 2174-81
2. Berghausen EJ, Balogh K, Landis WJ, Lee DD, Wright AM. Cervical myelopathy attributable to pseudogout. Case report with radiologic. histologic and crystallographic observations. Clin Orthop Relat Res. 1987. 214: 217-21
3. Bywaters EG, Hamilton EB, Williams R. The spine in idiopathic haemochromatosis. Ann Rheum Dis. 1971. 30: 453-65
4. Caird J, Roberts G, Young S, Brett F. Calcium pyrophosphate dihydrate crystal deposition disease: A case of cervical myelopathy in an elderly woman. J Neurol Neurosurg Psychiatry. 1999. 67: 547-8
5. Canhão H, Fonseca JE, Leandro MJ, Romeu JC, Pimentão JB, Costa JT. Cross-sectional study of 50 patients with calcium pyrophosphate dihydrate crystal arthropathy. Clin Rheumatol. 2001. 20: 119-22
6. Chen CF, Chang MC, Wang ST, Liu CL, Chen W. Calcium pyrophosphate dihydrate crystal deposition disease in cervical radiculomyelopathy. J Chin Med Assoc. 2003. 66: 256-9
7. Ellman MH, Vazquez T, Ferguson L, Mandel N. Calcium pyrophosphate deposition in ligamentum flavum. Arthritis Rheum. 1978. 21: 611-3
8. Gomez H, Chou SM. Myeloradiculopathy secondary to pseudogout in the cervical ligamentum flavum: Case report. Neurosurgery. 1989. 25: 298-302
9. Haraguchi K, Yamaki T, Kurokawa Y, Ohtaki M, Ibayashi Y, Uede T. A case of calcification of the cervical ligamentum flavum. No Shinkei Geka. 1996. 24: 69-73
10. Hirabayashi S. Ossification of the ligamentum flavum. Spine Surg Relat Res. 2017. 1: 158-63
11. Kawano N, Matsuno T, Miyazawa S, Iida H, Yada K, Kobayashi N. Calcium pyrophosphate dihydrate crystal deposition disease in the cervical ligamentum flavum. J Neurosurg. 1988. 68: 613-20
12. Kawano N, Matsuno T, Miyazawa S, Uchiyama H, Ohtaka H, Mii K. New knowledge on the calcium pyrophosphate dihydrate (CPPD) crystal deposition disease in the cervical ligamentum flavum. No Shinkei Geka. 1987. 15: 181-90
13. Kawano N, Yoshida S, Ohwada T, Yada K, Sasaki K, Matsuno T. Cervical radiculomyelopathy caused by deposition of calcium pyrophosphate dihydrate crystals in the ligamenta flava. Case report. J Neurosurg. 1980. 52: 279-83
14. Kimura R, Miyakoshi N, Kobayashi T, Kudo D, Shimada Y. Acute exacerbation of cervical myelopathy caused by pseudogout of the cervical ligamentum flavum after cervical spinal cord injury: A case report. Spinal Cord Ser Cases. 2020. 6: 98
15. Kobayashi T, Miyakoshi N, Abe T, Abe E, Kikuchi K, Noguchi H. Acute neck pain caused by pseudogout attack of calcified cervical yellow ligament: A case report. J Med Case Rep. 2016. 10: 133
16. Kobayashi T, Miyakoshi N, Konno N, Ishikawa Y, Noguchi H, Shimada Y. Age-related prevalence of periodontoid calcification and its associations with acute cervical pain. Asian Spine J. 2018. 12: 1117-22
17. Kohn NN, Hughes RE, McCarty DJ, Faires JS. The significance of calcium phosphate crystals in the synovial fluid of arthritic patients: The “pseudogout syndrome” II. Identification of crystals. Ann Intern Med. 1962. 56: 738-45
18. Liao JH, Huang KC, Hsieh CT, Sun JM. Cervical myeloradiculopathy as an initial presentation of pseudogout. Neurosciences (Riyadh). 2021. 26: 93-6
19. Lin SH, Hsieh ET, Wu TY, Chang CW. Cervical myelopathy induced by pseudogout in ligamentum flavum and retroodontoid mass: A case report. Spinal Cord. 2006. 44: 692-4
20. Lu YH, Lin HH, Chen HY, Chou PH, Wang ST, Liu CL. Multilevel calcium pyrophosphate dihydrate deposition in cervical ligamentum flavum: Clinical characteristics and imaging features. BMC Musculoskelet Disord. 2021. 22: 929
21. Morino T, Ogata T, Horiuchi H, Yamaoka S, Fukuda M, Miura H. Eight years of follow-up after laminectomy of calcium pyrophosphate crystal deposition in the cervical yellow ligament of patient with Coffin-Lowry syndrome: A case report. Medicine (Baltimore). 2016. 95: e4468
22. Moshrif A, Laredo JD, Bassiouni H, Abdelkareem M, Richette P, Rigon MR. Spinal involvement with calcium pyrophosphate deposition disease in an academic rheumatology center: A series of 37 patients. Semin Arthritis Rheum. 2019. 48: 1113-26
23. Muthukumar N, Karuppaswamy U. Tumoral calcium pyrophosphate dihydrate deposition disease of the ligamentum flavum. Neurosurgery. 2003. 53: 103-8
24. Mwaka ES, Yayama T, Uchida K, Kobayashi S, Kokubo Y, Nakajima H. Calcium pyrophosphate dehydrate crystal deposition in the ligamentum flavum of the cervical spine: Histopathological and immunohistochemical findings. Clin Exp Rheumatol. 2009. 27: 430-8
25. Nagashima C, Takahama M, Shibata T, Nakamura H, Okada K, Morita H. Calcium pyrophosphate dihydrate deposits in the cervical ligamenta flava causing myeloradiculopathy. J Neurosurg. 1984. 60: 69-80
26. Ogata M, Ishikawa K, Ohira T. Cervical myelopathy in pseudogout. Case report. J Bone Joint Surg Am. 1984. 66: 1301-3
27. Reginato AJ, Tamesis E, Netter P. Familial and clinical aspects of calcium pyrophosphate deposition disease. Curr Rheumatol Rep. 1999. 1: 112-20
28. Richette P, Bardin T, Doherty M. An update on the epidemiology of calcium pyrophosphate dihydrate crystal deposition disease. Rheumatology (Oxford). 2009. 48: 711-5
29. Sairyo K, Biyani A, Goel V, Leaman D, Booth R, Thomas J. Pathomechanism of ligamentum flavum hypertrophy: A multidisciplinary investigation based on clinical, biomechanical, histologic, and biologic assessments. Spine (Phila Pa 1976). 2005. 30: 2649-56
30. Sekijima Y, Yoshida T, Ikeda S. CPPD crystal deposition disease of the cervical spine: A common cause of acute neck pain encountered in the neurology department. J Neurol Sci. 2010. 296: 79-82
31. Steinbach LS, Resnick D. Calcium pyrophosphate dihydrate crystal deposition disease revisited. Radiology. 1996. 200: 1-9
32. Sushil P, Anant K. Ossified-calcified ligamentum flavum causing dorsal cord compression with computed tomography-magnetic resonance imaging features. Surg Neurol. 1994. 41: 441-2
33. Takahashi T, Hanakita J, Minami M. Pathophysiology of calcification and ossification of the ligamentum flavum in the cervical spine. Neurosurg Clin N Am. 2018. 29: 47-54
34. Yamagami T, Kawano N, Nakano H. Calcification of the cervical ligamentum flavum case report. Neurol Med Chir (Tokyo). 2000. 40: 234-8
35. Yang X, Matsuda K, Bialek P, Jacquot S, Masuoka HC, Schinke T. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin-Lowry syndrome. Cell. 2004. 117: 387-98
36. Zitnan D, Sit’aj S. Chondrocalcinosis articularis Section L clinical and radiological study. Ann Rheum Dis. 1963. 22: 142-52