- Department of Neurosurgery, Mansoura University Hospital, Dakahliya, Egypt.
- Department of Clinical Pathology, Faculty of Medicine, Mansoura University, Dakahliya, Egypt.
- Department of Neurology, Mansoura University Hospital, Mansoura, Dakahliya, Egypt.
Correspondence Address:
Hany A. Fikry Eldawoody
Department of Neurology, Mansoura University Hospital, Mansoura, Dakahliya, Egypt.
DOI:10.25259/SNI_784_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hany A. Fikry Eldawoody1, Mohammed Abdel Bari Mattar1, Abeer Mesbah2, Ashraf Zaher3, Mohammed Elsherif3. Can brain natriuretic peptide, S100b, and interleukin-6 prognosticate the neurological consequences in Egyptian patients presented with supratentorial intracerebral hemorrhage?. 22-Dec-2020;11:460
How to cite this URL: Hany A. Fikry Eldawoody1, Mohammed Abdel Bari Mattar1, Abeer Mesbah2, Ashraf Zaher3, Mohammed Elsherif3. Can brain natriuretic peptide, S100b, and interleukin-6 prognosticate the neurological consequences in Egyptian patients presented with supratentorial intracerebral hemorrhage?. 22-Dec-2020;11:460. Available from: https://surgicalneurologyint.com/surgicalint-articles/10485/
Abstract
Background: Biomarkers in supratentorial intracerebral hemorrhage (SICH) enhance the prognosis of the disease. This study aimed to assess the prognosticative grade of S100 calcium-binding protein B (S100B), interleukin-6 (IL-6), and the pro-brain natriuretic peptide (pro-BNP) in SICH outcome prediction.
Methods: Blood samples of 50 SICH patients were analyzed for the biomarkers. The patients were classified into two groups with and without intraventricular hemorrhage (IVH). The following scales including Glasgow Coma Score (GCS), the Barthel index (BI), intracerebral hemorrhage (ICH) score, ICH volume, National Institutes of Health Stroke Scale (NIHSS), Modified Rankin Score (mRS), and length of stay were used to evaluate the severity.
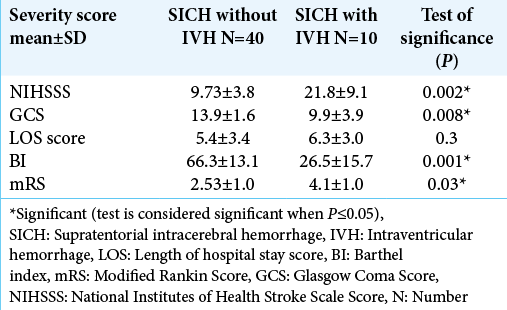
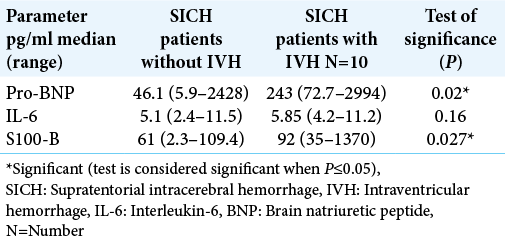
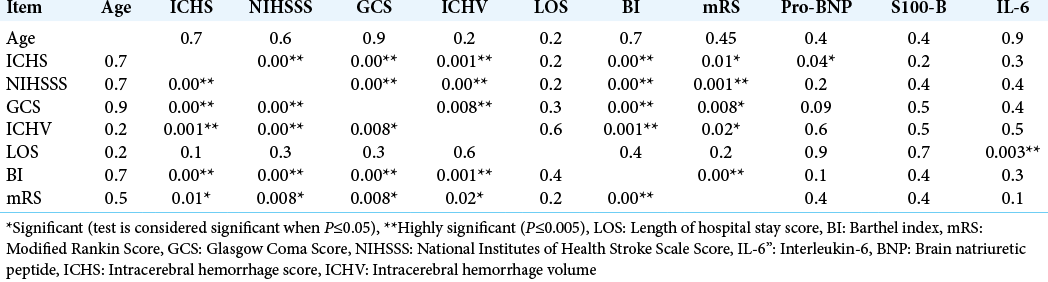
Results: The severity scores (NIHSS, GCS, BI, mRI) were significantly higher in SICH patients with IVH versus SICH patients without IVH (P = 0.002, 0.008, 0.001, and 0.03, respectively). Serum levels for a pro-BNP and S100b are significantly higher in SICH patients with IVH versus SICH patients without IVH (P = 0.02 and 0.027, respectively). Multivariate correlations between demographic (age), biomarkers panel (IL-6, S100b, and proBNP), and clinical and severity scores (ICH score, ICH volume, length of hospital stay [LOS], BI, mRS, GCS, and NIHSSS) in all studied patients showed a highly significant correlation between ICH score and pro-BNP (P = 0.04). There was a highly significant correlation between LOS and IL-6 (P = 0.003).
Conclusion: Pro-BNP, IL-6, and S100b are greatly associated with the presence of IVH that, in turn, correlated well with poor clinical outcome measures.
Keywords: Interleukin-6, Outcome, Pro-brain natriuretic peptide, S100 calcium-binding protein b, Supratentorial intracerebral hemorrhage
INTRODUCTION
A relatively common and overwhelming disease is the primary supratentorial intracerebral hemorrhage (SICH) accompanied by variable prognosis despite the great advancement in its related neurological and neurosurgical management.[
Elevated serum level of interleukin-6 (IL-6) is known to be associated with a variety of brain pathologies including ischemic stroke,[
In this study, we assess the predictive value of the biomarker panel of S100b, IL-6, and pro-BNP, in the functional outcome after SICH, rather than conventional clinical and/or radiological methods.
MATERIALS AND METHODS
Participants
The present prospective study included 50 patients with SICH who were admitted to the neurosurgery/neurology departments over a 12-month period at Mansoura Emergency University Hospital, Egypt. The diagnosis was based on clinical assessment and computed tomographic (CT) head scanning immediately after the onset of the condition. All patients were assessed by taking a medical history, history of previous cerebrovascular strokes, hypertension, diabetes mellitus, and renal or hepatic disorders.
Ethical approval
A consent whether informed or written was obtained from each patient and the ethical committee also approved the study.
Clinical assessment scales
Clinical examination besides the neurological severity scales for the assessment of functional outcome at the discharge time was evaluated by Glasgow Coma Score (GCS), the Barthel index (BI), intracerebral hemorrhage score (ICHS), National Institutes of Health Stroke Scale (NIHSS), and Modified Rankin Score (mRS). GCS, BI, NIHSS, mRS, and ICHS results were tabulated. The ICHS is a validated 6-point score to evaluate risk in patients with SICH and includes initial GCS, hematoma location, and volume, whether intraventricular hematoma is present or not, and the age.[
Radiology evaluation
Within the initial 24 h after hospital admission, the studied group of patients was scanned using brain computed tomography and checked for hemorrhagic volume (cm3), presence of intraventricular hemorrhage (IVH), and midline shift (MLS). On discharge from Mansoura University Hospital, we used the following functional assessment scales; BI, length of hospital stay (LOS), and mRS, by a blinded observer evaluation method as they are the best for biomarker panel data. All studied patients showed CT-proven supratentorial ICH before taking the blood samples. Regarding the CT scans evaluation, a blinded neuroradiologist used the well-known simplified ellipsoid volume equation method described by Kothari et al.[
Laboratory assessments
About 8 ml venous blood was withdrawn from each patient and divided as follows: 2 ml into EDTA tube for complete blood picture, 1.8 ml into the citrated tube for prothrombin time and APTT, and the remaining blood into the plain tube to get sera for routine investigations and the remaining sera were divided into three aliquots which were stored frozen at –20°C till the time of assay of specific investigations: S100 protein, IL-6, and N-terminal-pro-BNP. The routine laboratory investigations included complete blood picture, random blood sugar, liver, kidney function tests, and coagulation tests: PT and APTT. All routine laboratory chemical tests were done by fully automated chemistry analyzer Cobas c 311 (Roche Diagnostic GmbH Mannheim, Germany). A complete blood picture was done by Cell Dyn 1800, Abbott, USA. Coagulation tests were done by Siemens reagents using the Coatron Coagulometer, Germany. Quantitative determination of IL-6 was done by the enzyme-linked immunosorbent assay (ELISA) technique using RayBio Kit, Cat # ELH-IL6-001, USA.[
Statistical analysis
The mean and standard deviation were used for the description of continuous variables, while percentages were used for categorical variables. The linear regression curve was used to express the correlation of CT outcomes to the biomarkers panel. The relationship between the biochemical markers panel and functional patient scores was assessed by the linear regression analysis for the BI. The mRS was evaluated as a dichotomous outcome, while the BI was evaluated as a continuous variable. Logistic regression analysis was done for mRS. Multivariate assumptions were used. The two-way interactions between covariates were used for additive effects. The tolerance and variance inflation tests were used to show the collinearity between the predictors. The SAS software version 9.3 or JMP 7.0.1 was used for the execution of those statistics.
RESULTS
General characteristics
The studied patients’ group comprised 50 patients with predominant male gender, with male-to-female ratio of 16:9 ≅ 1.7:1 patient. The mean age (in years) of the studied group was 60.7 ± 11.5. The studied SICH patients were subdivided into two groups based on the presence or absence of IVH.
Severity scores results
The severity scores (NIHSS, GCS, BI, and mRS) were significantly higher in SICH patients with IVH when compared with SICH patients without IVH (P = 0.002, 0.008, 0.001, and 0.03, respectively), while the LOS score did not show any statistical significance [
Laboratory results
Serum levels for a panel of blood biomarkers (pro-BNP and S100b) were significantly higher in SICH patients with IVH when compared with SICH patients without IVH (P = 0.02 and 0.027, respectively). The IL-6 did not show any statistical significance [
Correlations between clinical data, laboratory results, and severity scores
Multivariate correlations between demographic (age), biomarkers panel (IL-6, S100b, and pro-BNP), and clinical and severity scores (ICH score, ICH volume, LOS, BI, mRS, GCS, and NIHSSS) in all studied patients showed a highly significant correlation between ICH score and proBNP (P = 0.04). Moreover, there was a highly significant correlation between LOS and IL-6 (P = 0.003) [
DISCUSSION
Many studies have shown that there is a correlation between the biochemical marker panels and the prognosis in various acute onset brain lesion pathogenesis, such as ischemic stroke,[
CONCLUSION
Pro-BNP, IL-6, and S100b are greatly associated with the presence of IVH that correlated well with poor clinical outcome measures by NIHSSS, GCS, BI, and mRS. Moreover, these contribute to the prognostic biomarkers data over the severity scales that integrate both the clinical and radiographic characteristics. Nevertheless, those laboratory biomarkers added more prognostic value when conjoined with clinical severity scores, especially ICH score and proBNP. Further investigation of serial serum biomarkers measurements could be of value over a prolonged period, especially with the addition of cognitive function evaluation.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abilleira S, Montaner J, Molina CA, Monasterio J, Castillo J, Alvarez-Sabín J. Matrix metalloproteinase-9 concentration after spontaneous intracerebral hemorrhage. J Neurosurg. 2003. 99: 65-70
2. Abraha HD, Butterworth RJ, Bath PM, Wassif WS, Garthwaite J, Sherwood RA. Serum S-100 protein, relationship to clinical outcome in acute stroke. Ann Clin Biochem. 1997. 34: 366-70
3. Akdemir G, Luer MS, Dujovny M, Misra M. Intraventricular atrial natriuretic peptide for acute intracranial hypertension. Neurol Res. 1997. 19: 515-20
4. Allard L, Lescuyer P, Burgess J, Leung KY, Ward M, Walter N. ApoC-I and ApoC-III as potential plasmatic markers to distinguish between ischemic and hemorrhagic stroke. Proteomics. 2004. 4: 2242-51
5. Bauer J, Herrmann F. Interleukin-6 in clinical medicine. Ann Hematol. 1991. 62: 203-10
6. Biberthaler P, Mussack T, Wiedemann E, Kanz KG, Mutschler W, Linsenmaier U. Rapid identification of high-risk patients after minor head trauma (MHT) by assessment of S-100B: Ascertainment of a cut-off level. Eur J Med Res. 2002. 7: 164-70
7. Broderick J, Connolly S, Feldmann E, Hanley D, Kase C, Krieger D. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: A guideline from the American heart association/American stroke association stroke council, high blood pressure research council, and the quality of care and outcomes in research interdisciplinary working group. Stroke. 2007. 38: 2001-23
8. Büttner T, Weyers S, Postert T, Sprengelmeyer R, Kuhn W. S-100 protein: Serum marker of focal brain damage after ischemic territorial MCA infarction. Stroke. 1997. 28: 1961-5
9. Chaudhry SR, Stoffel-Wagner B, Kinfe TM, Güresir E, Vatter H, Dietrich D. Elevated systemic IL-6 levels in patients with aneurysmal subarachnoid hemorrhage is an unspecific marker for Post-SAH Complications. Int J Mol Sci. 2017. 18: 2580
10. Delgado P, Sabin JA, Santamarina E, Molina CA, Quintana M, Rosell A. Plasma S100B level after acute spontaneous intracerebral hemorrhage. Stroke. 2006. 37: 2837-39
11. Dziedzic T, Bartus S, Klimkowicz A, Motyl M, Slowik A, Szczudlik A. Intracerebral hemorrhage triggers interleukin-6 and interleukin-10 release in blood. Stroke. 2002. 33: 2334-5
12. Ekmektzoglou KA, Xanthos T, Papadimitriou L. Biochemical markers (NSE, S-100, IL-8) as predictors of neurological outcome in patients after cardiac arrest and return of spontaneous circulation. Resuscitation. 2007. 75: 219-28
13. 13. Elting JW, Sulter GA, Kaste M, Lees KR, Diener HC, Hommel M. AMPA antagonist ZK200775 in patients with acute ischemic stroke: Possible glial cell toxicity detected by monitoring of S-100B serum levels. Stroke. 2002. 33: 2813-8
14. Epstein M, Loutzenhiser R, Friedland E, Aceto RM, Camargo MJ, Atlas SA. Relationship of increased plasma atrial natriuretic factor and renal sodium handling during immersion-induced central hypervolemia in normal humans. J Clin Invest. 1987. 79: 738-45
15. Fang HY, Ko WJ, Lin CY. Plasma interleukin 11 levels correlate with outcome of spontaneous intracerebral hemorrhage. Surg Neurol. 2005. 64: 511-8
16. Floras JS. Sympathoinhibitory effects of atrial natriuretic factor in normal humans. Circulation. 1990. 81: 1860-73
17. Foerch C, Wunderlich MT, Dvorak F, Humpich M, Kahles T, Goertler M. Elevated serum S100B levels indicate a higher risk of hemorrhagic transformation after thrombolytic therapy in acute stroke. Stroke. 2007. 38: 2491-5
18. Fukui S, Nawashiro H, Otani N, Ooigawa H, Toyooka T, Tsuzuki N. Focal brain edema and natriuretic peptides in patients with subarachnoid hemorrhage. Acta Neurochir Suppl. 2003. 86: 489-91
19. Garibi J, Bilbao G, Pomposo I, Hostalot C. Prognostic factors in a series of 185 consecutive spontaneous supratentorial intracerebral haematomas. Br J Neurosurg. 2002. 16: 355-61
20. Hemphill JC, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: A simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001. 32: 891-7
21. Iida H, Iida M, Takenaka M, Oda A, Uchida M, Fujiwara H. The effects of alpha-human atrial natriuretic peptide and milrinone on pial vessels during blood-brain barrier disruption in rabbits. Anesth Analg. 2001. 93: 177-82
22. James ML, Blessing R, Phillips-Bute BG, Bennett E, Laskowitz DT. S100B and brain natriuretic peptide predict functional neurological outcome after intracerebral haemorrhage. Biomarkers. 2009. 14: 388-94
23. James ML, Blessing R, Bennett E, Laskowitz DT. Apolipoprotein E modifies neurological outcome by affecting cerebral edema but not hematoma size after intracerebral hemorrhage in humans. J Stroke Cerebrovasc Dis. 2009. 18: 144-9
24. James ML, Warner DS, Laskowitz DT. Preclinical models of intracerebral hemorrhage: A translational perspective. Neurocrit Care. 2008. 9: 139-52
25. Jönsson H, Johnsson P, Birch-Iensen M, Alling C, Westaby S, Blomquist S. S100B as a predictor of size and outcome of stroke after cardiac surgery. Ann Thorac Surg. 2001. 71: 1433-7
26. Juvela S. Risk factors for impaired outcome after spontaneous intracerebral hemorrhage. Arch Neurol. 1995. 52: 1193-200
27. Kanner AA, Marchi N, Fazio V, Mayberg MR, Koltz MT, Siomin V. Serum S100beta: A noninvasive marker of blood-brain barrier function and brain lesions. Cancer. 2003. 97: 2806-13
28. Koenig MA, Puttgen HA, Prabhakaran V, Reich D, Stevens RD. B-type natriuretic peptide as a marker for heart failure in patients with acute stroke. Intensive Care Med. 2007. 33: 1587-93
29. Kokocinska D, Wieczorek P, Partyka R, Jarzab J, Jałowiecki P, Sikora J. The diagnostic utility of S-100B protein and TPA in patients with ischemic stroke. Neuro Endocrinol Lett. 2007. 28: 693-8
30. Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996. 27: 1304-5
31. Laragh JH. Atrial natriuretic hormone, the renin-aldosterone axis, and blood pressure-electrolyte homeostasis. N Engl J Med. 1985. 313: 1330-40
32. Laskowitz DT, Blessing R, Floyd J, White WD, Lynch JR. Panel of biomarkers predicts stroke. Ann N Y Acad Sci. 2005. 1053: 30
33. Lyden P, Raman R, Liu L, Grotta J, Broderick J, Olson S. NIHSS training and certification using a new digital video disk is reliable. Stroke. 2005. 36: 2446-9
34. Lynch JR, Blessing R, White WD, Grocott HP, Newman MF, Laskowitz DT. Novel diagnostic test for acute stroke. Stroke. 2004. 35: 57-63
35. Mårtenson ED, Hansson LO, Nilsson B, von Schoultz E, Brahme EM, Ringborg U. Serum S-100b protein as a prognostic marker in malignant cutaneous melanoma. J Clin Oncol. 2001. 19: 824-31
36. McGirt MJ, Blessing R, Nimjee SM, Friedman AH, Alexander MJ, Laskowitz DT. Correlation of serum brain natriuretic peptide with hyponatremia and delayed ischemic neurological deficits after subarachnoid hemorrhage. Neurosurgery. 2004. 54: 1369-74
37. Mizukoshi G, Katsura K, Katayama Y. Urinary 8-hydroxy-2’-deoxyguanosine and serum S100beta in acute cardioembolic stroke patients. Neurol Res. 2005. 27: 644-6
38. Mukoyama M, Nakao K, Hosoda K, Suga S, Saito Y, Ogawa Y. Brain natriuretic peptide as a novel cardiac hormone in humans, Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J Clin Invest. 1991. 87: 1402-12
39. Mukoyama M, Nakao K, Saito Y, Ogawa Y, Hosoda K, Suga S. Increased human brain natriuretic peptide in congestive heart failure. N Engl J Med. 1990. 323: 757-8
40. Nakagawa K, Yamaguchi T, Seida M, Yamada S, Imae S, Tanaka Y. Plasma concentrations of brain natriuretic peptide in patients with acute ischemic stroke. Cerebrovasc Dis. 2005. 19: 157-64
41. Nir A, Lindinger A, Rauh M, Bar-Oz B, Laer S, Schwachtgen L. NT-pro-B-type natriuretic peptide in infants and children: Reference values based on combined data from four studies. Pediatr Cardiol. 2009. 30: 3-8
42. Nogami M, Shiga J, Takatsu A, Endo N, Ishiyama I. Immunohistochemistry of atrial natriuretic peptide in brain infarction. Histochem J. 2001. 33: 87-90
43. 43. Pettigrew LC, Kasner SE, Gorman M, Atkinson RP, Funakoshi Y, Ishibashi H. Effect of arundic acid on serum S-100beta in ischemic stroke. J Neurol Sci. 2006. 251: 57-61
44. Reynolds MA, Kirchick HJ, Dahlen JR, Anderberg JM, McPherson PH, Nakamura KK. Early biomarkers of stroke. Clin Chem. 2003. 49: 1733-9
45. Sanchez-Peña P, Pereira AR, Sourour NA, Biondi A, Lejean L, Colonne C. S100B as an additional prognostic marker in subarachnoid aneurysmal hemorrhage. Crit Care Med. 2008. 36: 2267-73
46. Sodeck GH, Domanovits H, Sterz F, Schillinger M, Losert H, Havel C. Can brain natriuretic peptide predict outcome after cardiac arrest? An observational study. Resuscitation. 2007. 74: 439-45
47. Stranjalis G, Korfias S, Psachoulia C, Kouyialis A, Sakas DE, Mendelow AD. The prognostic value of serum S-100B protein in spontaneous subarachnoid haemorrhage. Acta Neurochir (Wien). 2007. 149: 231-8
48. Suzuki S, Tanaka K, Suzuki N. Ambivalent aspects of interleukin-6 in cerebral ischemia: Inflammatory versus neurotrophic aspects. J Cereb Blood Flow Metab. 2009. 29: 464-79
49. Sviri GE, Feinsod M, Soustiel JF. Brain natriuretic peptide and cerebral vasospasm in subarachnoid hemorrhage, Clinical and TCD correlations. Stroke. 2000. 31: 118-22
50. Sviri GE, Shik V, Raz B, Soustiel JF. Role of brain natriuretic peptide in cerebral vasospasm. Acta Neurochir (Wien). 2003. 145: 851-60
51. Sviri GE, Soustiel JF, Zaaroor M. Alteration in brain natriuretic peptide (BNP) plasma concentration following severe traumatic brain injury. Acta Neurochir (Wien). 2006. 148: 529-33
52. Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974. 2: 81-4
53. Valli N, Gobinet A, Bordenave L. Review of 10 years of the clinical use of brain natriuretic peptide in cardiology. J Lab Clin Med. 1999. 134: 437-44
54. Weglewski A, Ryglewicz D, Mular A, Juryńczyk J. Changes of protein S100B serum concentration during ischemic and hemorrhagic stroke in relation to the volume of stroke lesion. Neurol Neurochir Pol. 2005. 39: 310-7
55. Woiciechowsky C, Schöning B, Cobanov J, Lanksch WR, Volk HD, Döcke WD. Early IL-6 plasma concentrations correlate with severity of brain injury and pneumonia in brain-injured patients. J Trauma. 2002. 52: 339-45
56. Yarlagadda S, Rajendran P, Miss JC, Banki NM, Kopelnik A, Wu AH. Cardiovascular predictors of in-patient mortality after subarachnoid hemorrhage. Neurocrit Care. 2006. 5: 102-7