- Department of Neurosurgery, Kovai Medical Center and Hospitals, Coimbatore, Tamil Nadu, India.
- Department of Neuro-Imaging and Stroke Interventions, Kovai Medical Center and Hospitals, Coimbatore, Tamil Nadu, India.
- Department of Neuropathology, Kovai Medical Center and Hospitals, Coimbatore, Tamil Nadu, India.
Correspondence Address:
Sagar Amrutlal Ghodasara
Department of Neuropathology, Kovai Medical Center and Hospitals, Coimbatore, Tamil Nadu, India.
DOI:10.25259/SNI_410_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sagar Amrutlal Ghodasara1, Rohit Balasubramanian1, Shriram Varadharajan2, P. S. Shobhanaa3. Cardiac phoenix in the brain-occult intracranial hemorrhagic metastases from completely resected atrial myxoma. 11-Nov-2020;11:383
How to cite this URL: Sagar Amrutlal Ghodasara1, Rohit Balasubramanian1, Shriram Varadharajan2, P. S. Shobhanaa3. Cardiac phoenix in the brain-occult intracranial hemorrhagic metastases from completely resected atrial myxoma. 11-Nov-2020;11:383. Available from: https://surgicalneurologyint.com/surgicalint-articles/10390/
Abstract
Background: Cardiac myxomas are sporadic in nature and can often recur with a frequency of 3%, especially in middle-aged women, and 22% of the cases account to a part of Carney complex. Complete surgical removal of the myxoma is usually curative. Recurrence has been related with partial surgical excision, multicentricity, and embolism of tumor fragments.
Case Description: We report a case of a patient with single brain metastases due to tumor embolization, from a cardiac myxoma operated prior. This case is exclusive, as tumor embolization from atrial myxoma to the cerebral cortex can be possible, within a short duration. In our case, the patient was evaluated with a magnetic resonance imaging brain and a solitary hemorrhagic lesion in the eloquent cerebral cortex was observed. To determine the primary etiology, the diagnosis of probable metastases was thought of, and a thorough workup was planned. Surprisingly, no primary lesion was detected, and as a histological diagnosis was required, he underwent a navigation-guided excisional biopsy of lesion. The biopsy was indicative of a metastatic deposit from an atrial myxoma.
Conclusion: In eloquent cortex lesions, gross total resection is challenging for a neurosurgeon especially when the patient has no significant neurological deficits. Timely gross total resection of a solitary metastatic lesion can improve the patient’s outcome and can enhance early recovery with less or no morbidity.
Keywords: Cerebral metastasis, Eloquent visual cortex lesion, Excisional biopsy, Left atrial myxoma, Magnetic resonance imaging, Positron emission tomography
INTRODUCTION
Cardiac myxoma is the most common benign heart tumor, frequently found in the left atrium in about 86% of the cases.[
CASE HISTORY
A 63-year-old male was admitted with chief complaints of moderate left-sided hemicranial headache for 1 month which was on and off in nature and not associated with any aura. The headache progressively increased over 7 days and was associated with vomiting. He presented with an unsteady gait along with giddiness. He underwent an excision of a left atrial myxoma, a year ago and was on regular follow-up with an uneventful cardiac evaluation. A cardiologist’s evaluation with a 2D ECHO showed no residual lesion in heart. A magnetic resonance imaging brain [
He underwent left parieto-occipital craniotomy with navigation-guided excision of the lesion. Intraoperatively, there was a yellowish-gray soft lesion (6.0 × 4.4 × 5.9 cm) extending up to the left occipital horn of lateral ventricle with moderate vascularity. There were 2–3 satellite lesions observed in the hemorrhagic and necrosed tumor bed. Postoperatively, he improved well neurologically, with an improvement of gait imbalance.
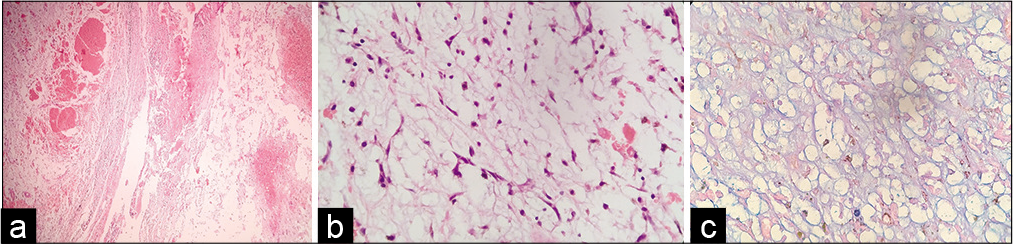
HPE report [
Neuroimaging
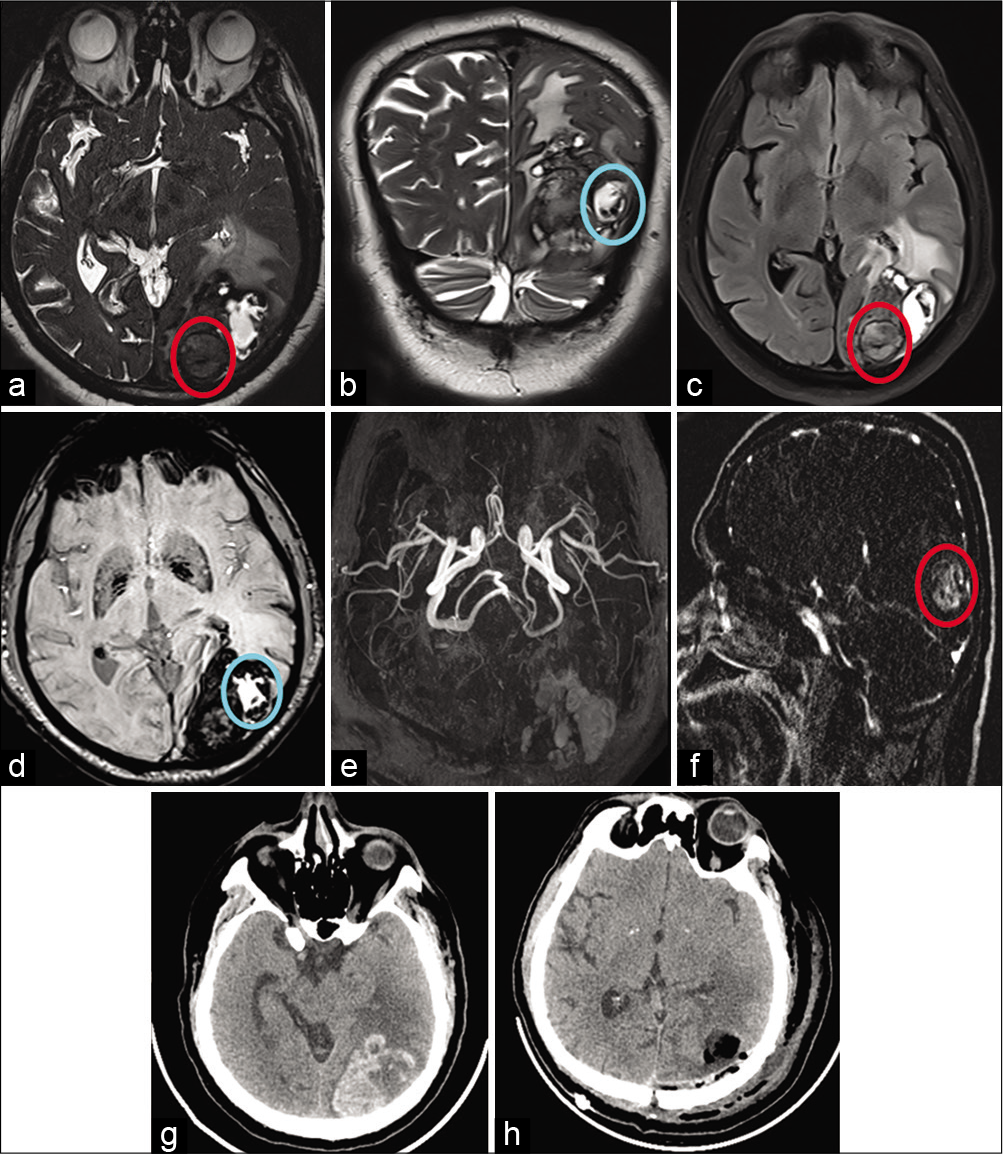
Figure 1:
Top row – Axial and coronal T2-weighted (a and b) images show large heterogeneous hemorrhagic lesion in the left occipital region with perilesional edema and mass effect. Differential soft tissue is noted along posterior aspect (red circle) on T2 and FLAIR (c) with hypointensity and blooming on SWI (d) as compared to adjacent hematoma (blue circle) showing hyperintensity with peripheral blooming. Bottom row – Axial TOF MRA images (e) show no obvious vascular abnormality and postcontrast sagittal subtracted T1 MPRAGE images (f) show intense enhancement within differential soft tissue (red circle on f). Preoperative plain CT (g) shows hemorrhagic lesion and postoperative CT shows resection cavity with air pockets and near complete resection of lesion (h).
Histopathology
DISCUSSION
Cardiac myxoma is the most common benign heart tumor, that is, sporadic (93% of cases) with a female preponderance.
Cardiac myxoma can also be a component of an autosomal dominant syndrome called Carney complex. Carney complex is characterized by spotty pigmentation (blue nevi and lentigines), myxomas (cardiac, cutaneous, and mammary), endocrine overactivity (Cushing’s syndrome and acromegaly), testicular tumors, and Schwannomas.[
Metastatic lesions have been reported to be diagnosed up to 8 years later than the primary lesion. In the majority of cases, the cerebral arteries, including the retinal arteries, are affected. However, it has been postulated that tumor tissues may grow into the walls of the vessels causing focal disruption of the internal elastic lamina, providing a nidus for cerebral hemorrhage and subsequent growth of metastatic tumor tissue.[
The fate of the tumor fragment, which embolizes to the cerebral vessel within the central nervous system, still remains controversial. Two late complications have been reported: either a tumor fragment may grow and present as an expanding mass lesion, or it may develop a vascular aneurysm at the site of the embolus.[
As the natural history of this disease is unknown, the standard of care for these intraparenchymal lesions remains controversial. Thus, a high index of suspicion is required so that diagnosis can be effectively made and patients can be put under surveillance.
Our patient underwent a surgery for myxoma 1 year back. As our patient had a cortical bleed in an accessible location, excision biopsy was possible, which helped us arrive at a diagnosis. In patients who present with multifocal intracerebral hemorrhages, biopsy from a noneloquent region is mandatory to establish the cause for bleed.
CONCLUSION
A standard of care in the management of cardiac myxoma patients with cerebral metastases is yet to be established. While surgery may be appropriate in cases with one or two isolated brain metastases, palliative radiotherapy could be administered to patients with multiple brain metastases. Vigorous workup must be pursued if the patient becomes symptomatic yet again or develops fresh neurological manifestations.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Acebo E, Val-Bernal JF, Gómez-Román JJ, Revuelta JM. Clinicopathologic study and DNA analysis of 37 cardiac myxomas: A 28-year experience. Chest. 2003. 123: 1379-85
2. Bazin A, Peruzzi P, Baudrillard JC, Pluot M, Rousseaux P. Cardiac myxoma with cerebral metastases. Neurochirurgie. 1987. 33: 487-9
3. Burke AP, Virmani R. Cardiac myxoma. A clinicopathologic study. Am J Clin Pathol. 1993. 100: 671-80
4. Ekinci EI, Donnan GA. Neurological manifestations of cardiac myxoma: A review of the literature and report of cases. Intern Med J. 2004. 34: 243-9
5. Hwang BJ, Connelly MM, Lev MH. Distinctive MR imaging appearance of hemorrhagic cerebral aneurysms associated with atrial myxoma. AJR Am J Roentgenol. 2001. 177: 925-7
6. Jean WC, Walski-Easton SM, Nussbaum ES. Multiple intracranial aneurysms as delayed complications of an atrial myxoma: Case report. Neurosurgery. 2001. 49: 200-2
7. Keeling IM, Oberwalder P, Anelli-Monti M, Schuchlenz H, Demel U, Tilz GP. Cardiac myxomas: 24 years of experience in 49 patients. Eur J Cardiothorac Surg. 2002. 22: 971-7
8. Kumar A, Sachdev A, Singh R, Lehl SS, D’Cruz S, Mahapatra M. Left atrial myxoma presenting as pseudobulbar palsy. Neurol India. 2002. 50: 219-21
9. Price DL, Harris JL, New PF, Cantu RC. Cardiac myxoma. A clinicopathologic and angiographic study. Arch Neurol. 1970. 23: 558-67
10. Rodrigues D, Matthews N, Scoones D, Aziz F, Nath F. Recurrent cerebral metastasis from a cardiac myxoma: Case report and review of literature. Br J Neurosurg. 2006. 20: 318-20
11. Sakamoto H, Sakamaki T, Sumino H, Sawada Y, Sato H, Sato M. Production of endothelin-1 and big endothelin-1 by human cardiac myxoma cells--implications of the origin of myxomas. Circ J. 2004. 68: 1230-2
12. Shapiro LM. Cardiac tumours: Diagnosis and management. Heart. 2001. 85: 218-22
13. Shimono T, Makino S, Kanamori Y, Kinoshita T, Yada I. Left atrial myxomas. Using gross anatomic tumor types to determine clinical features and coronary angiographic findings. Chest. 1995. 107: 674-9
14. Stratakis CA, Kirschner LS, Carney JA. Carney complex: Diagnosis and management of the complex of spotty skin pigmentation, myxomas, endocrine overactivity, and schwannomas. Am J Med Genet. 1998. 80: 183-5
15. Yoon DH, Roberts WC. Sex distribution in cardiac myxomas. Am J Cardiol. 2002. 90: 563-5