- Department of Neurosurgery, Showa University Hospital, Shinagawa-ku, Yokohama City, Japan
- Department of Neurosurgery, Showa University Fujigaoka Hospital, Yokohama City, Japan
- Department of Neuroendovascular Therapy, Yokohama Municipal Citizen’s Hospital, Yokohama City, Japan
Correspondence Address:
Hiroki Nagatsuka, Department of Neurosurgery, Showa University Hospital, Shinagawa-ku, Japan.
DOI:10.25259/SNI_700_2022
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hiroki Nagatsuka1, Yuma Miki2, Yoshiaki Tetsuo3, Hajime Yabuzaki2, Sadayoshi Nakayama2, Yuko Tanaka2, Yoshikazu Matsuda2, Tomoyuki Tsumoto2, Tomoaki Terada2, Tohru Mizutani1. Carotid artery stenting for stenosis after carotid artery replacement: An illustrative case report. 13-Jan-2023;14:8
How to cite this URL: Hiroki Nagatsuka1, Yuma Miki2, Yoshiaki Tetsuo3, Hajime Yabuzaki2, Sadayoshi Nakayama2, Yuko Tanaka2, Yoshikazu Matsuda2, Tomoyuki Tsumoto2, Tomoaki Terada2, Tohru Mizutani1. Carotid artery stenting for stenosis after carotid artery replacement: An illustrative case report. 13-Jan-2023;14:8. Available from: https://surgicalneurologyint.com/surgicalint-articles/12106/
Abstract
Background: There are few reports on the treatment of carotid artery stenosis after arterial vessel replacement. We report and discuss an illustrative case of carotid artery stenting (CAS) performed for stenosis after carotid artery replacement.
Case Description: A woman in her 20s experienced injury to the right carotid artery during an operation for removal of a carotid body tumor 6 years before presentation. The right common carotid artery and internal carotid artery were replaced with an artificial vessel graft at that time. Intraluminal stenosis in the graft was not identified 3 years after surgery; however, 4 years after surgery, stenosis was recognized at the non-anastomotic site inside the artificial vessel graft. Subsequently, antiplatelet therapy was initiated. The stenosis was noted to progress gradually in follow-up appointments. Therefore, we decided to intervene because of the patient’s young age and the risk of long-term hemodynamic stress. Angiography revealed pseudo-occlusion in the artificial vessel. Percutaneous transluminal angioplasty was performed for stenosis with distal protection; subsequently, CAS was performed. The patient was discharged without neurological deficits 4 days after the operation, and no apparent restenosis was observed as of the 1-year follow-up.
Conclusion: Stenosis after cervical artery replacement can be safely treated with CAS. Inflation pressure and stent should be selected according to the pathology of the stenosis.
Keywords: Artificial blood vessel, Carotid artery stenosis, Carotid artery stenting, Percutaneous transluminal angioplasty (PTA)
INTRODUCTION
Surgical interventions for stenosis after carotid artery replacement have rarely been reported. In general, in-graft stenosis after arterial replacement tends to occur at the anastomotic site due to intimal thickening. In this article, we report a rare case of stenosis at the non-anastomotic site in the late stage after carotid artery replacement, which was successfully treated with carotid artery stenting (CAS).
CASE DESCRIPTION
A woman in her twenties underwent right carotid body tumor removal at a different hospital 6 years before presentation. During the operation, the right carotid artery was injured and the right common carotid artery and internal carotid artery were replaced with an artificial vessel comprised of expanded polytetrafluoroethylene (ePTFE, outer diameter of 4 mm). The patient was discharged without any neurological deficits. Magnetic resonance angiography (MRA) showed no obvious stenosis 2 years after the surgery [
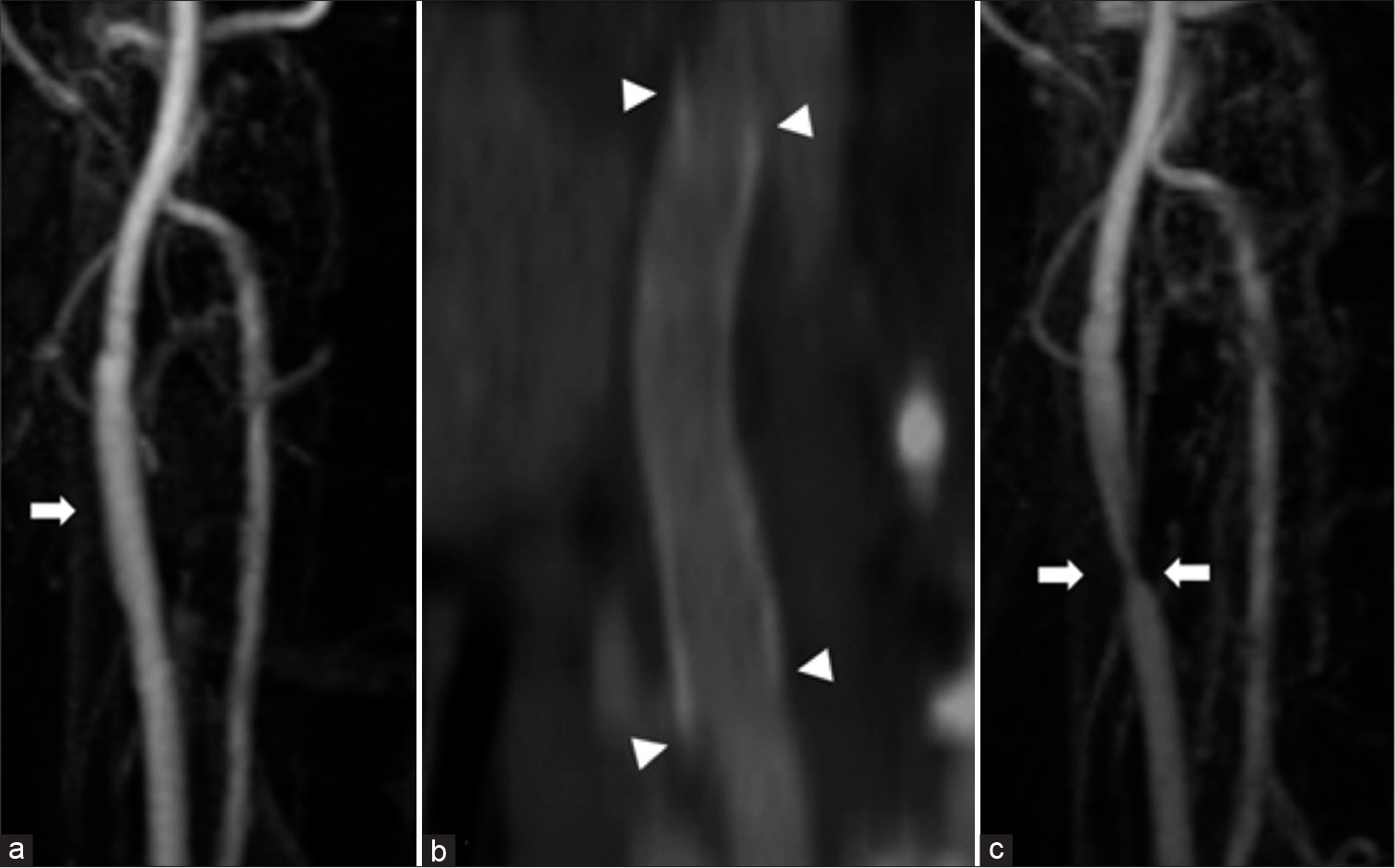
Figure 1:
(a) Carotid magnetic resonance angiography: 2 years after surgery. There is no obvious stenosis in the right carotid artery (arrow). (b) The right cervical computed tomography angiography: 3 years after surgery. There is no obvious stenosis in the artificial vessel at 3 years after surgery. The arrowhead indicates each end of the anastomosis. (c) Carotid magnetic resonance angiography: 4 years after surgery, The stenosis of the right carotid artery is observed (arrow).
Four years after the surgery, MRA showed suspicious stenosis in the artificial vessel [
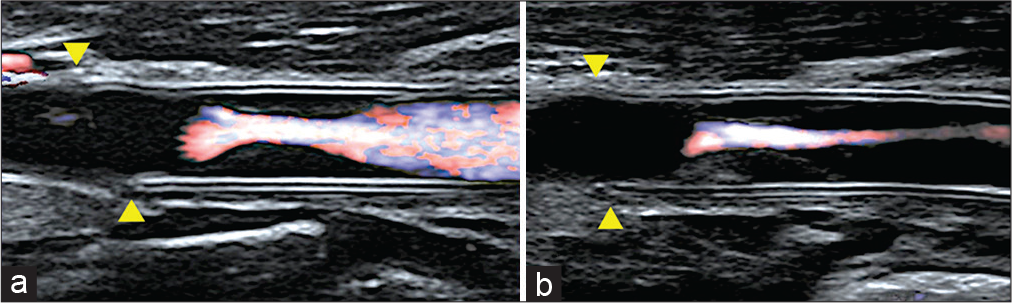
Figure 2:
Carotid artery echocardiography (a: 4 years after surgery, b: 6 years after surgery, arrow head: proximal anastomotic site). (a) The stenosis is recognized inside the vessel graft. The peak systolic velocity is 242 cm/s. (b) The stenosis is progressed as seen during the follow-up. The PSV is 255 cm/s. The lumen of the prosthesis shows isointense intimal thickening, and the stenosis is clearly progressive.
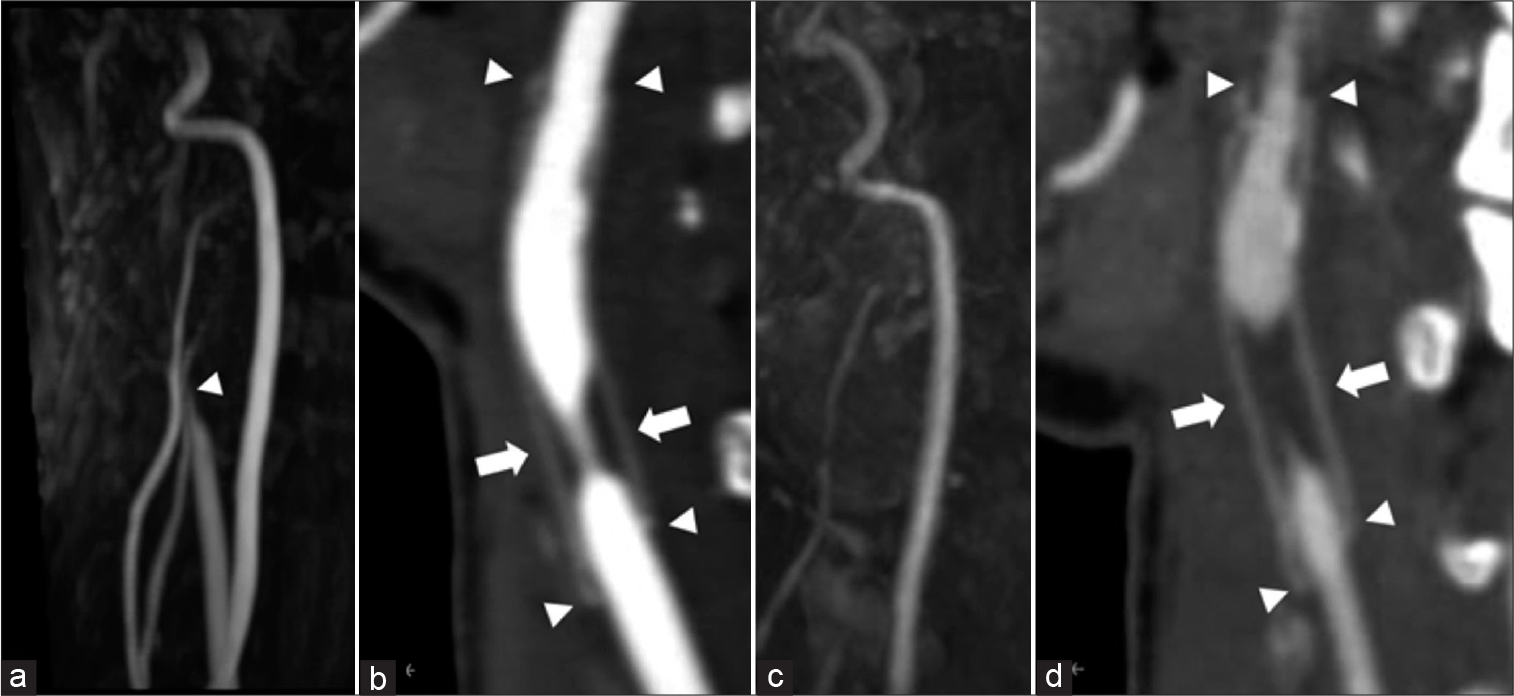
Figure 3:
(a) Carotid magnetic resonance angiography: 6 years after surgery. The right carotid artery is suspected obstruction (arrowhead). (b) The right cervical computed tomography angiography: 6 years after surgery. The arrowhead indicates each end of the anastomosis. Six years later, the stenosis is observed at the non-anastomotic area (arrow). (c) Carotid magnetic resonance angiography: Just before IVR. The right carotid artery was further poorly delineated. (d) The right cervical computed tomography angiography: Just before IVR. The stenosis progressed just before treatment (arrow). The arrowhead indicates each end of the anastomosis.
Although the patient was asymptomatic and CBF was maintained, we decided to perform endovascular revascularization with her informed consent because of the risk of long-term hemodynamic stress and the possibility of adhesion after the previous surgery. We planned percutaneous transluminal angioplasty (PTA) with a non-compliant balloon to fully dilate the stenosis and then deployed an open-cell stent to prevent restenosis. The common carotid artery was difficult to detect on the cervical MRA [
Interventional radiology
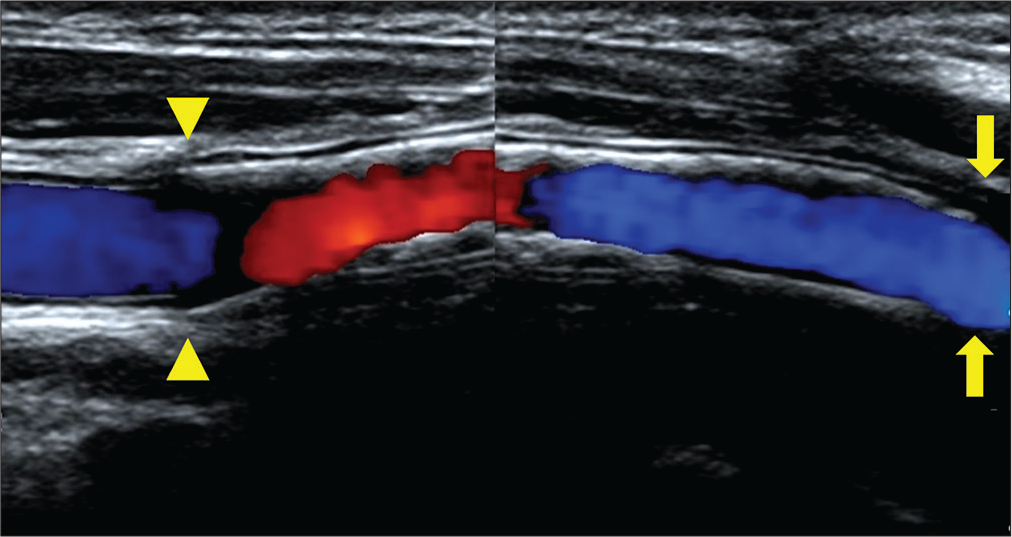
The patient was treated under local anesthesia and near-infrared spectroscopy (NIRS) monitoring. An 8Fr long sheath was inserted into the left femoral artery. Heparin was administered at a dose of 5000 units and added to maintain an ACT ≥250. An 8Fr OPTIMO (Tokai Medical Products, Aichi) guiding catheter with a balloon was navigated to the right common carotid artery. Common carotid angiography showed pseudo-occlusion of the stenosis [
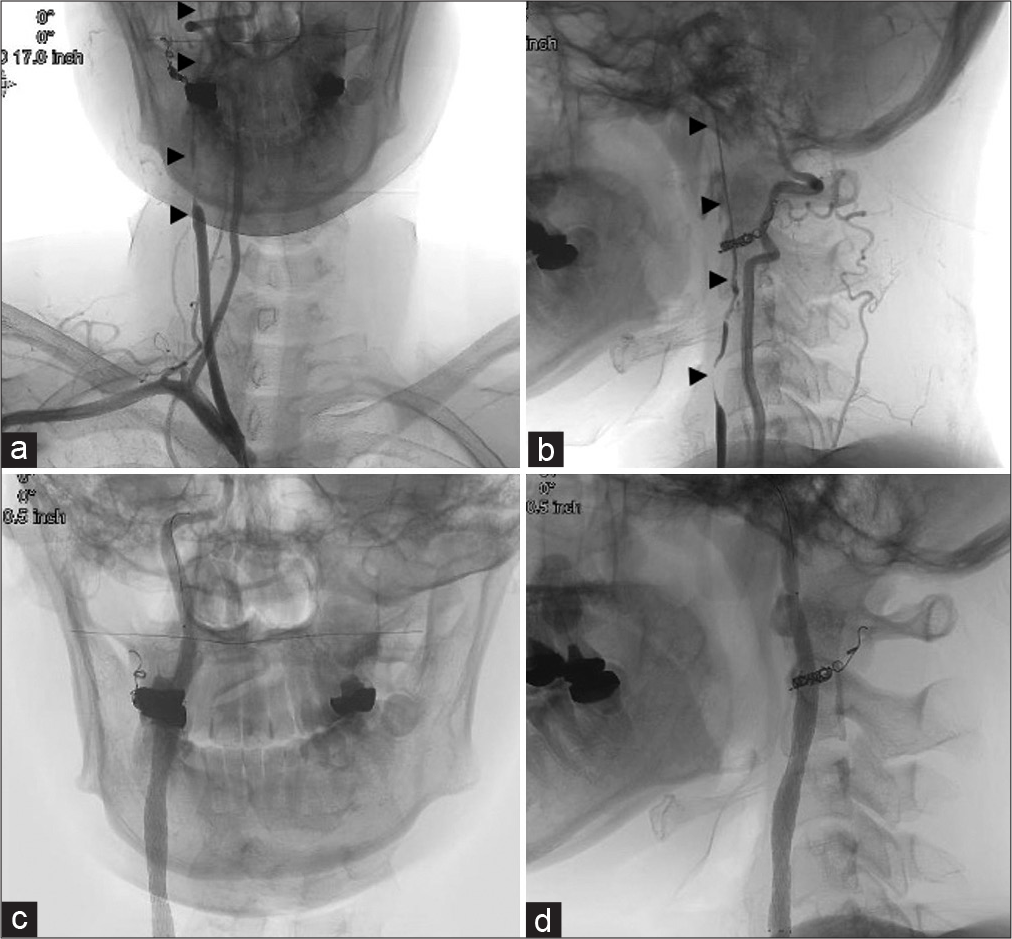
Figure 4:
(a and b) Preoperative brachiocephalic angiography (a: AP view, b: Lateral view). (b) The right common and internal carotid artery was pseudo-occluded up to the intracranial area (arrow head). (c and d) Postoperative common carotid arteriography (c: AP view, d: Lateral view), (d) The stenosis was well dilated with the stent.
DISCUSSION
In this report, we presented an illustrative case of cervical prosthetic stenosis. It is generally believed that stenosis tends to occur at the anastomotic site of an artificial vessel. However, in this case, the stenosis was at the non-anastomotic site. CAS was performed for the lesion, and a good outcome was obtained. Here, we review the literature on stenosis in artificial blood vessel and its treatment.
Artificial blood vessel
Teflon ePTFE artificial blood vessels are currently used in most clinical cases. This product is made by processing resin and is considered to have better antithrombotic properties than Dacron artificial blood vessels. ePTFE is mainly used for peripheral vascular replacement, such as in shunt surgery for cyanotic heart disease and vascular access for hemodialysis.[
Stenosis in artificial blood vessel
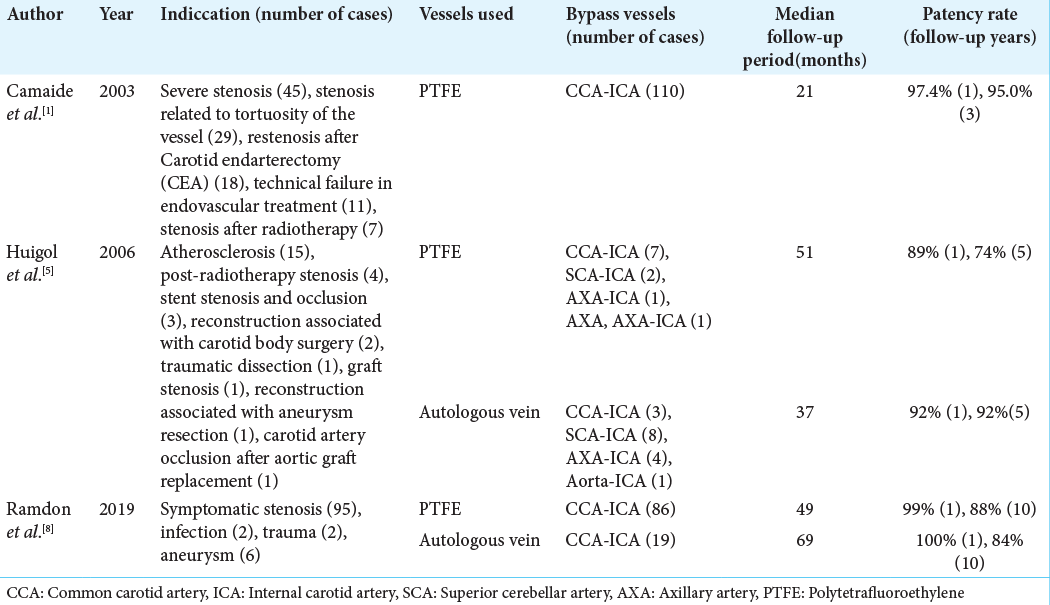
There have been several reports on the patency rate of artificial blood vessels after carotid artery bypass surgery using a polytetrafluoroethylene graft.
According to one study, the patency rate was almost 89–100% 1 year after surgery; however, this rate decreased over time, suggesting that the occurrence of stenosis and occlusion during the long-term period is not rare. Other reports have shown that the patency rate with artificial vessels was not significantly different from that of autologous veins.[
Additional reports have addressed the site of stenosis after replacement of the femoral artery with artificial grafts. Stenosis and occlusion are generally known to occur at the portion of the anastomosis between the artificial vessel and original vessel.[
Another interesting point of this case is that stenosis at the nonanastomotic site developed 4 years after the replacement. The main part of the stenosis was visualized as an isointense area on carotid ultrasonography and was thought to be fibrous tissue. The patient did not have any medical problems or connective tissue diseases that could cause carotid artery stenosis. Studies of grafts at the femoral artery have reported that intimal damage was caused by external physical stimuli, resulting in prosthetic vessel stenosis.[
Treatment of stenosis in artificial blood vessel
There are few reports on the treatment of cervical artery stenosis after replacement with artificial vessels. Conservative treatment for asymptomatic stenosis is usually performed with antiplatelet medication; however, there are few reports of surgical intervention.[
In this case, based on ultrasonography findings, the stenosis was thought to be mainly caused by fibrous components; therefore, the lesion was expected to be a comparatively hard component. Hence, although there was a risk of damaging the vessel graft itself, a PTA balloon with a slightly larger diameter than that of the artificial vessel was selected. In fact, the first PTA at 8 atm was not sufficient, and the second dilatation (performed at 14 atm) was required. Inflation pressure may need to be determined according to the pathology of the stenosis. Regarding the selection of the stent, an open-cell stent with a high radial force is recommended. Moreover, considering the low compliance of the prosthesis, we need to be mindful of potential migration of debris distally during the procedure.
CONCLUSION
Herein, we reported a rare case of delayed stenosis at the nonanastomotic site of an artificial vessel after carotid artery replacement. Based on our experience, stenosis after cervical artery replacement can be safely and effectively treated with CAS. PTA with a noncompliant balloon and implantation of an open-cell stent may be effective in improvement of the stenosis and preventing restenosis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Camaide C, Maher A, Ricco JB, Roumy J, Febrer G, Marchand C. Carotid bypass with polytetrafluoroethylene grafts: A study of 110 consecutive patients. J Vasc Surg. 2003. 38: 1031-8
2. Clowes AW, Gown AM, Hanson SR, Reidy MA. Mechanisms of arterial graft failure: Role of cellular proliferation in early healing of PTFE prosthesis. Am J Pathol. 1985. 118: 43-54
3. Cormier JM, Laurian C, Noel Y, Gigou F, Ricco JB, Fichelle JM. Aorto-femoral bypass with polytetrafluoroethylene prostheses: Preliminary results in 363 cases. Ann Vasc Surg. 1986. 1: 43-9
4. Fukushima H, Yao Y, Nagae T, Ishimaru S. Long-term results of femoropopliteal bypass with Weinkled polytetrafluoroethylene: Risk Factors for graft failures. Jpn J Vasc Surg. 2001. 10: 595-9
5. Huigol RL, Young J, Lemech L, Stephen MS, May J, Harris JP. Durability of autogenous vein and polytetrafluoroethylene bypass grafts to the carotid arteries. ANZ J Surg. 2006. 76: 878-81
6. Matsumoto H, Takemoto H, Nishiyama H, Tetsuo Y, Higashiue S, Nakao N. A case of percutaneous transluminal angioplasty and stenting for Dacron bypass graft stenosis with cerebral infarction. No Shinkei Geka. 2015. 43: 1011-8
7. Ogino H, Matsuda H, Minatoya K, Sasaki H, Tanaka H, Iba Y. Long-term degeneration of prosthetic vascular graft after aortic surgery. J Jpn Coll Angiol. 2011. 51: 47-52
8. Ramdon A, Martinez-Singh K, Hanath JC, Chang BB, Darling RC. Long term patency of venous and prosthetic conduits for ipsilateral internal carotid artery bypass. J Vasc Surg. 2019. 70: 1935-41
9. Ross R, Glomset J, Harker L. Response to injury and atherogenesis. Am J Pathol. 1977. 86: 675-84
10. Sato H, Okamura M, Ohta T. Long-term results of above-knee arterial revascularization with thin-walled, stretched polytetrafluoroethylene vascular graft. Jpn J Vasc Surg. 2001. 10: 527-30
11. Sise MJ, Ivy ME, Malanche R, Ranbarger KR. Polytetrafluoroethylene interposition grafts for carotid reconstructino. J Vasc Surg. 1992. 16: 601-8
12. Takagaki Y, Komai H, Juri M, Iwahashi M, Hayashi H, Yamamoto S. Localized stenosis in a non-anastomotic site of the graft after external iliac-common femoral artery crossover bypass using a 6mm Dacron graft. Jpn J Vasc. 2009. 18: 635-9
13. Takeda F, Yamamoto K, Sugimoto T, Kasuya S, Oguma F, Shinonaga M. Mid-term outcome of the hybrid PTFE vascular prostheses for the patients with arteriosclerotic occlusive disease. J Jpn Coll Angiol. 2003. 43: 771-3
14. Walpoth BH, Rogulenko R, Tikhvinskaia E, Gogolewski S, Schaffner T, Hess OM. Improvement of patency rate in heparin-coated small synthetic vascular grafts. Circulation. 1998. 98: II319-24