- Department of Orthopaedic Surgery, Rutgers-Robert Wood Johnson Medical School, New Brunswick, New Jersey, United States.
Correspondence Address:
Todd H. Alter, Department of Orthopaedic Surgery, Rutgers-Robert Wood Johnson Medical School, New Brunswick, New Jersey, United States.
DOI:10.25259/SNI_192_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Todd H. Alter, Thomas Helbig, Gino Chiappetta. Case report: Multiple sclerosis diagnosis after anterior lumbar interbody fusion and presumed COVID-19 infection. 31-Mar-2022;13:125
How to cite this URL: Todd H. Alter, Thomas Helbig, Gino Chiappetta. Case report: Multiple sclerosis diagnosis after anterior lumbar interbody fusion and presumed COVID-19 infection. 31-Mar-2022;13:125. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11494
Abstract
Background: Multiple sclerosis (MS) is an autoimmune demyelinating disease of the central nervous system that may present with a wide variety of clinical presentations. However, there can be substantial overlap between symptoms from MS and those caused by lumbar spondylosis and/or postviral plexopathies.
Case Description: A 33-year-old female with a history of an L5-S1 anterior lumbar interbody fusion and exposure to the SARS-CoV-2 virus developed postoperative worsening of her symptoms interpreted as “radiculopathy.” Despite a subsequent L5-S1 fusion, she continued to neurologically deteriorate and was ultimately diagnosed with MS.
Conclusion: The initial symptoms/signs of MS may mimic lumbar radiculopathy and or postviral plexopathy (i.e., due to recent COVID-19). This report should serve as a warning to future spinal surgeons to better differentiate between radicular and other “complaints,” sufficient to avoid unnecessary repeated spinal surgery.
Keywords: Anterior lumbar interbody fusion, COVID-19, Lumbar, Multiple sclerosis, Myelopathy, Spine, Radiculopathy
INTRODUCTION
Because multiple sclerosis (MS) can produce a wide array of neurological symptoms, it can mimic a variety of other conditions. These include myelopathy attributed to vertebral disc herniation, spinal stenosis, or spondylosis, and more recently COVID-19-related viral plexopathy.[
CASE
History
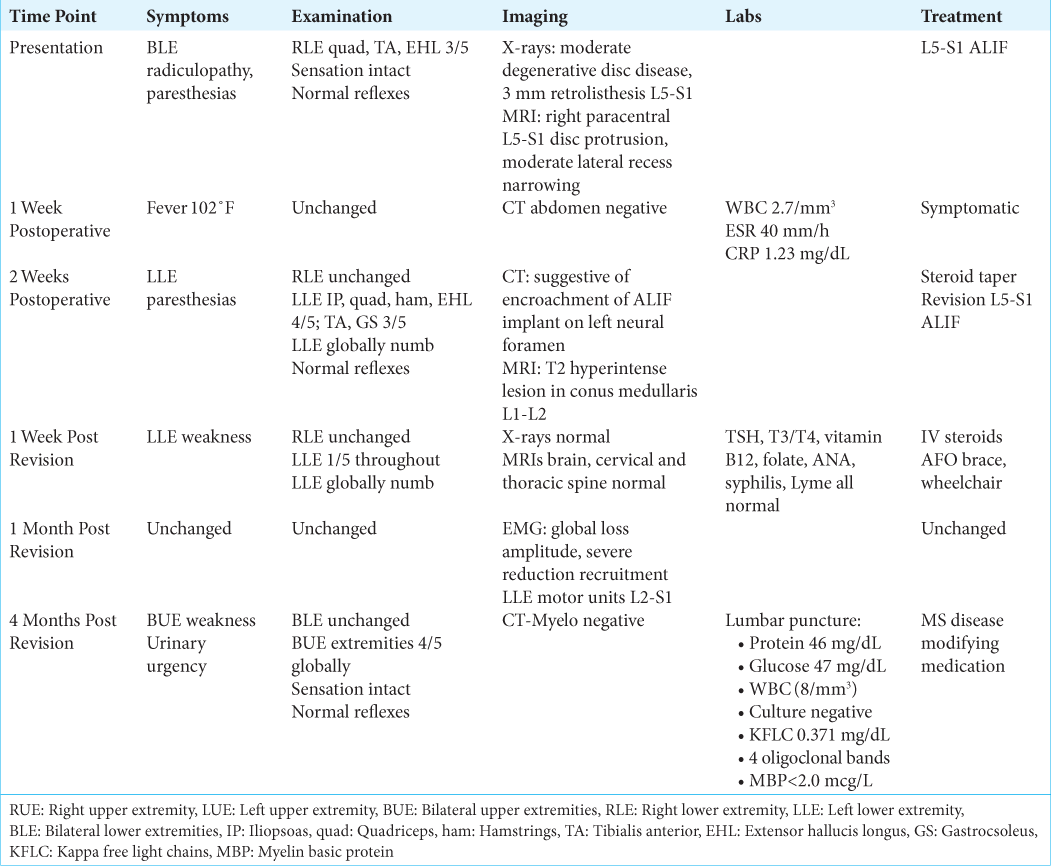
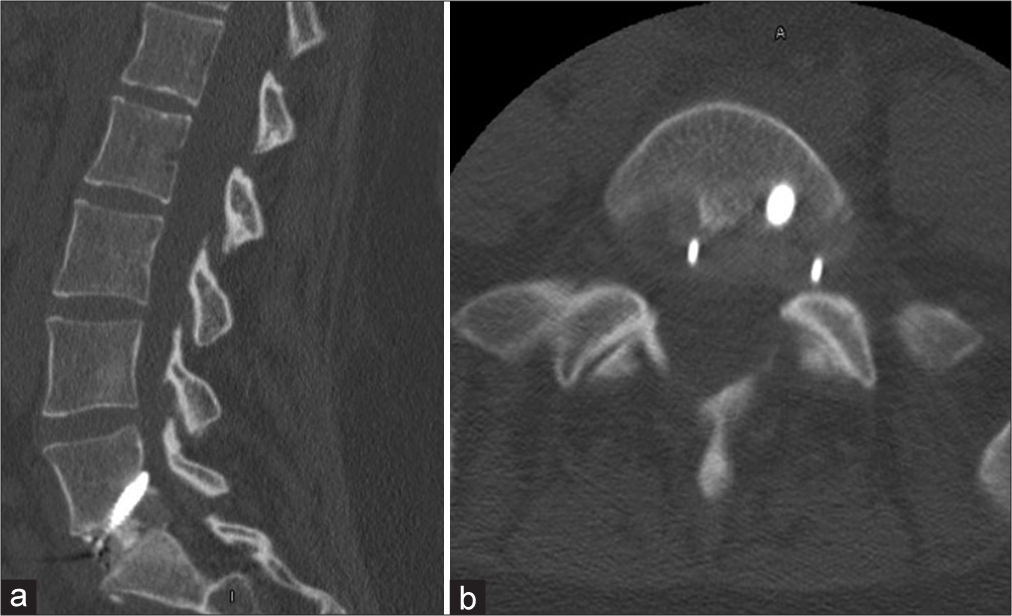
A 33-year-old female with a medical history of hypothyroidism, polycystic ovarian syndrome, pseudotumor cerebri, pacemaker placement for bradycardia, a gastric sleeve, and a C6-7 cervical disc replacement (1 year prior for myelopathy), presented with 2 years of low back pain/radiculopathy. On examination, she exhibited significant isolated weakness in her right lower extremity without sensory or reflex abnormalities [
Postoperative course
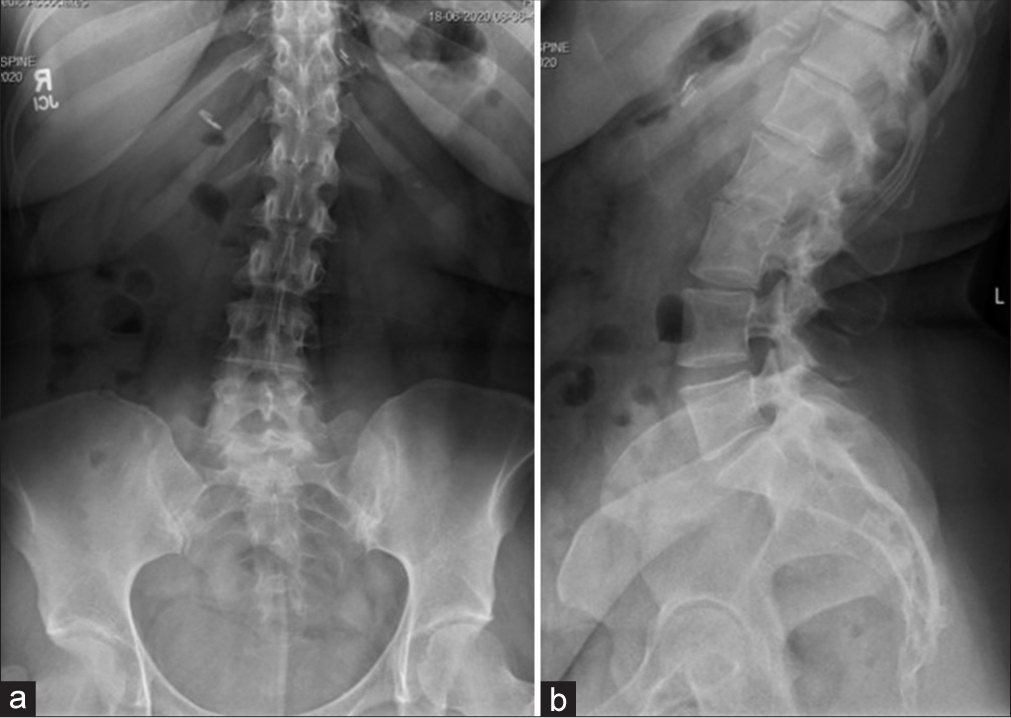
One week postoperatively, she had fevers, but a postoperative CT of the abdomen and laboratory studies were normal. Shortly thereafter, her husband tested positive for COVID-19; she was not tested at that time. Two weeks postoperatively, she complained of new left leg paresthesias (i.e., note her prior deficits were right-sided) with weakness and diffusely decreased sensation to light touch. She was given a steroid taper and sent for a lumbar CT scan that “suggested” encroachment on the left L5-S1 neural foramen by the L5-S1 ALIF [
Revision surgery followed by diagnosis of MS versus poly-sensory neuropathy
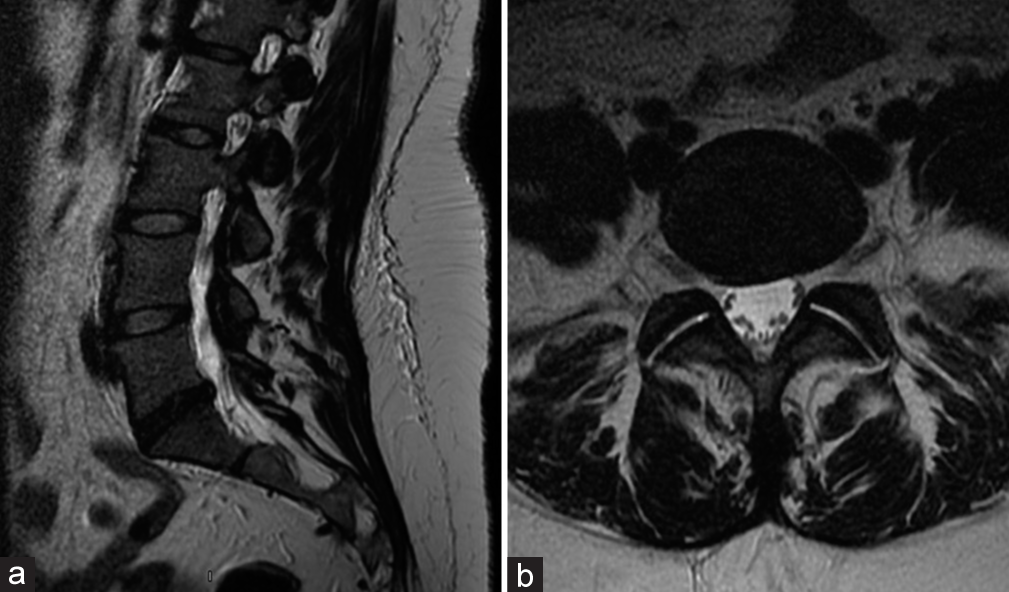
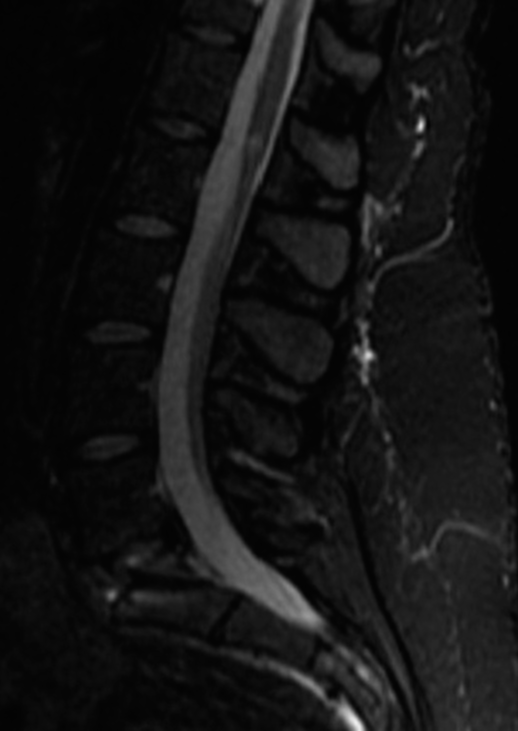
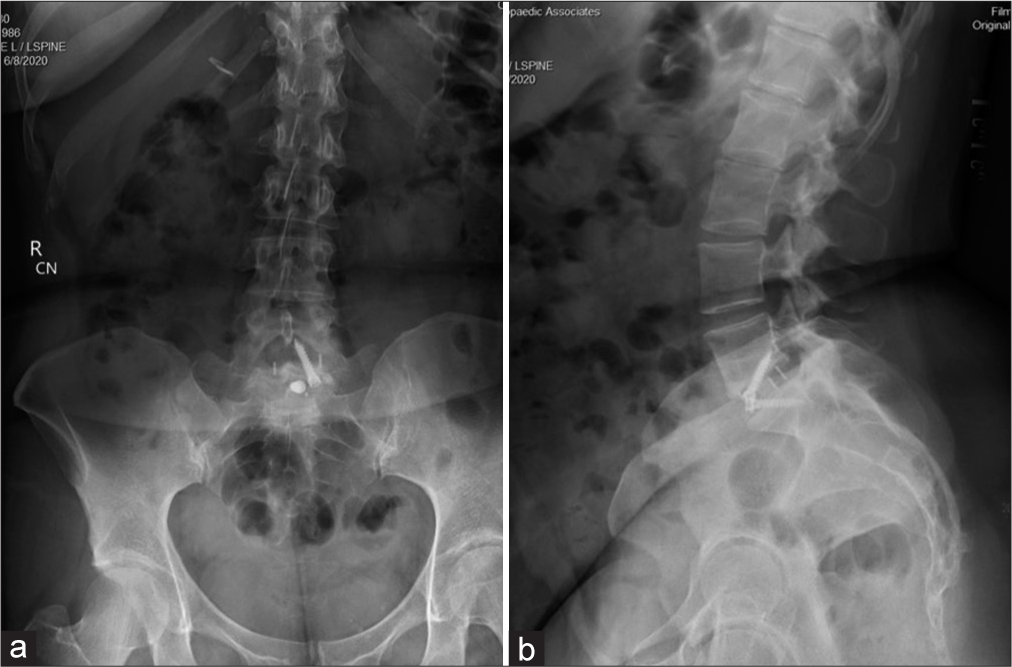
She then underwent a revision ALIF for cage repositioning. Postoperatively, her symptoms continued to worsen. MRIs of the cervical, thoracic spine, and brain were all normal along with multiple lab tests. She was sent home, but was now wheelchair bound. Repeat X-rays showed good location of the L5-S1 ALIF cage [
Delayed diagnosis of MS
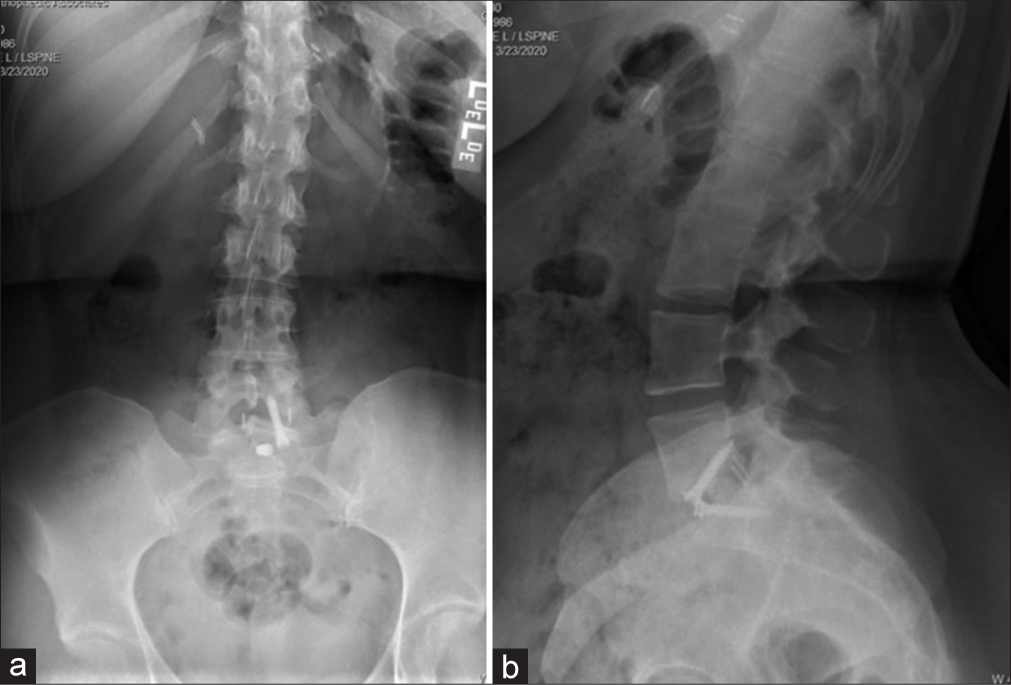
Four months later, now with additional bilateral upper and lower extremity weakness/sensory loss, and worsening urinary urgency with incomplete bladder emptying, she underwent a complete spinal Myelo-CT; it was negative. However, the cerebrospinal fluid obtained from the lumbar puncture showed findings consistent with MS (i.e., primary progressive subtype). She then began MS modifying therapy.
DISCUSSION
Diagnosis of MS versus spinal pathology and risks of spine surgery precipitating demyelinating events
The diagnosis of MS can easily be missed when patients present with myelopathic symptoms misinterpreted as “radiculopathy” secondary to spinal pathology/spondylotic disease. In these cases, patients’ failures to improve with surgery were ultimately attributed to the underlying diagnosis of MS and may result in unintended exacerbation of MS (i.e., precipitate demyelinating events).[
Increase in MS diagnoses related to COVID-19
Some have observed an increasing relationship between COVID-19 and the onset of MS (i.e., a spike in MS diagnoses).[
CONCLUSION
The diagnosis of MS can easily be missed when patients present with myelopathic symptoms misinterpreted as “radiculopathy” secondary to spinal pathology. Further, recent COVID-19 infections may pose triggers that potentiate the development of MS.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflict of interest.
References
1. Andersen O, Lygner PE, Bergström T, Andersson M, Vahlne A. Viral infections trigger multiple sclerosis relapses: A pros pective seroepidemiological study. J Neurol. 1993. 240: 417-22
2. D’hooghe MB, Nagels G, Bissay V, De Keyser J. Modifiable factors influencing relapses and disability in multiple sclerosis. Mult Scler. 2010. 16: 773-85
3. Möhn N, Konen FF, Pul R, Kleinschnitz C, Prüss H, Witte T. Experience in multiple sclerosis patients with COVID-19 and disease-modifying therapies: A review of 873 published cases. J Clin Med. 2020. 9: 4067
4. Moore L, Ghannam M, Manousakis G. A first presentation of multiple sclerosis with concurrent COVID-19 infection. eNeurologicalSci. 2021. 22: 100299
5. Palao M, Fernández-Díaz E, Gracia-Gil J, RomeroSánchez CM, Díaz-Maroto I, Segura T. Multiple sclerosis following SARS-CoV-2 infection. Mult Scler Relat Disord. 2020. 45: 102377
6. Sadeghmousavi S, Rezaei N. COVID-19 and multiple sclerosis: Predisposition and precautions in treatment. SN Compr Clin Med. 2020. 2: 1802-7
7. Satheesh NJ, Salloum-Asfar S, Abdulla SA. The potential role of COVID-19 in the pathogenesis of multiple sclerosis-a preliminary report. Viruses. 2021. 13: 2091
8. Yerneni K, Nichols N, Burke JF, Traynelis VC, Tan LA. Surgical management of patients with coexistent multiple sclerosis and cervical stenosis: A systematic review and meta-analysis. J Clin Neurosci. 2019. 65: 77-82
9. Young WF, Weaver M, Mishra B. Surgical outcome in patients with coexisting multiple sclerosis and spondylosis. Acta Neurol Scand. 1999. 100: 84-7
10. Zabalza A, Cárdenas-Robledo S, Tagliani P, Arrambide G, Otero-Romero S, Carbonell-Mirabent P. COVID-19 in multiple sclerosis patients: Susceptibility, severity risk factors and serological response. Eur J Neurol. 2021. 28: 3384-95